Metals and non-metals: Chemistry AQA: GCSE (9:1)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Metals
Elements which form positive ions
Non-metals
Elements which do not form positive ions
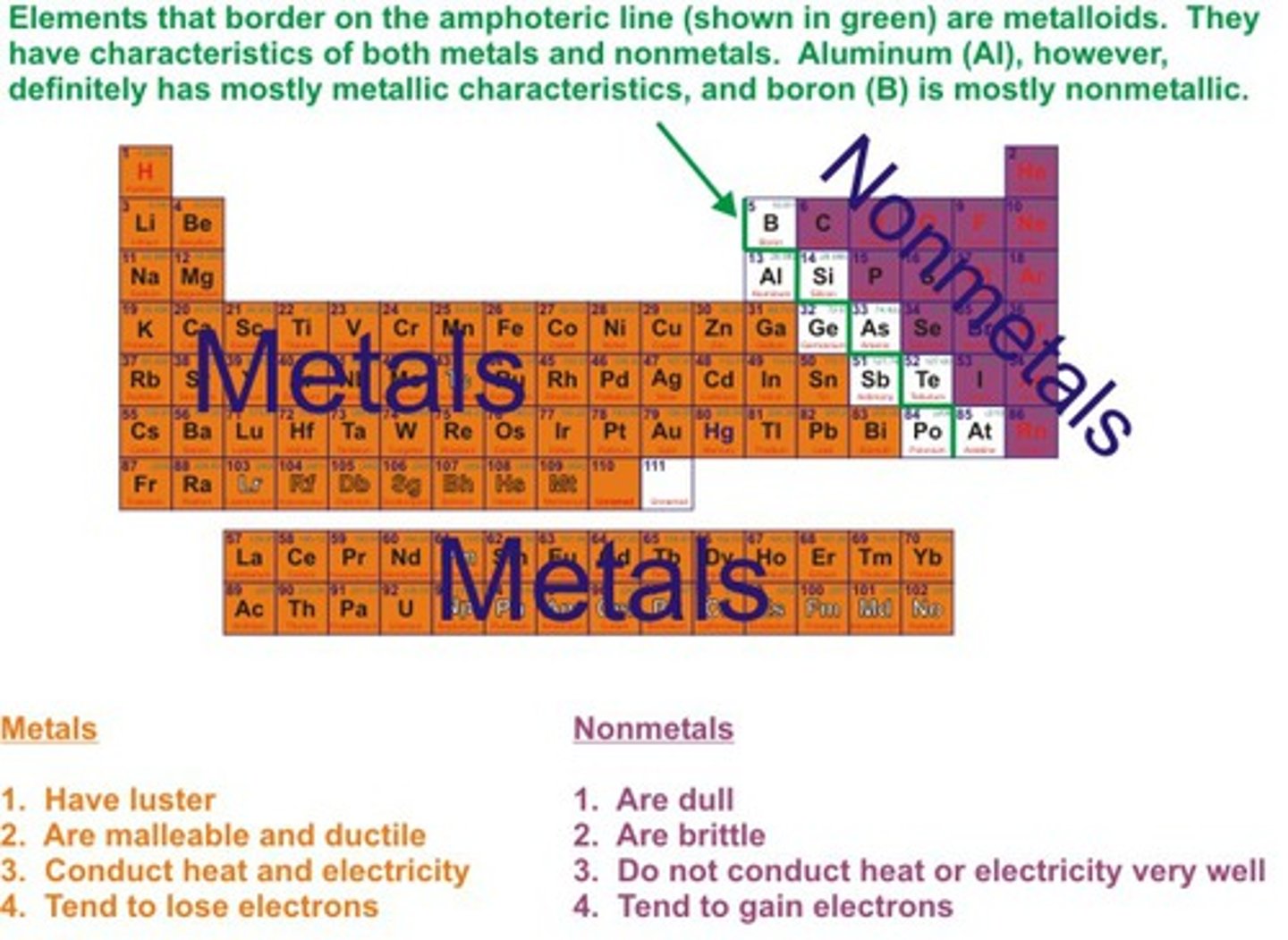
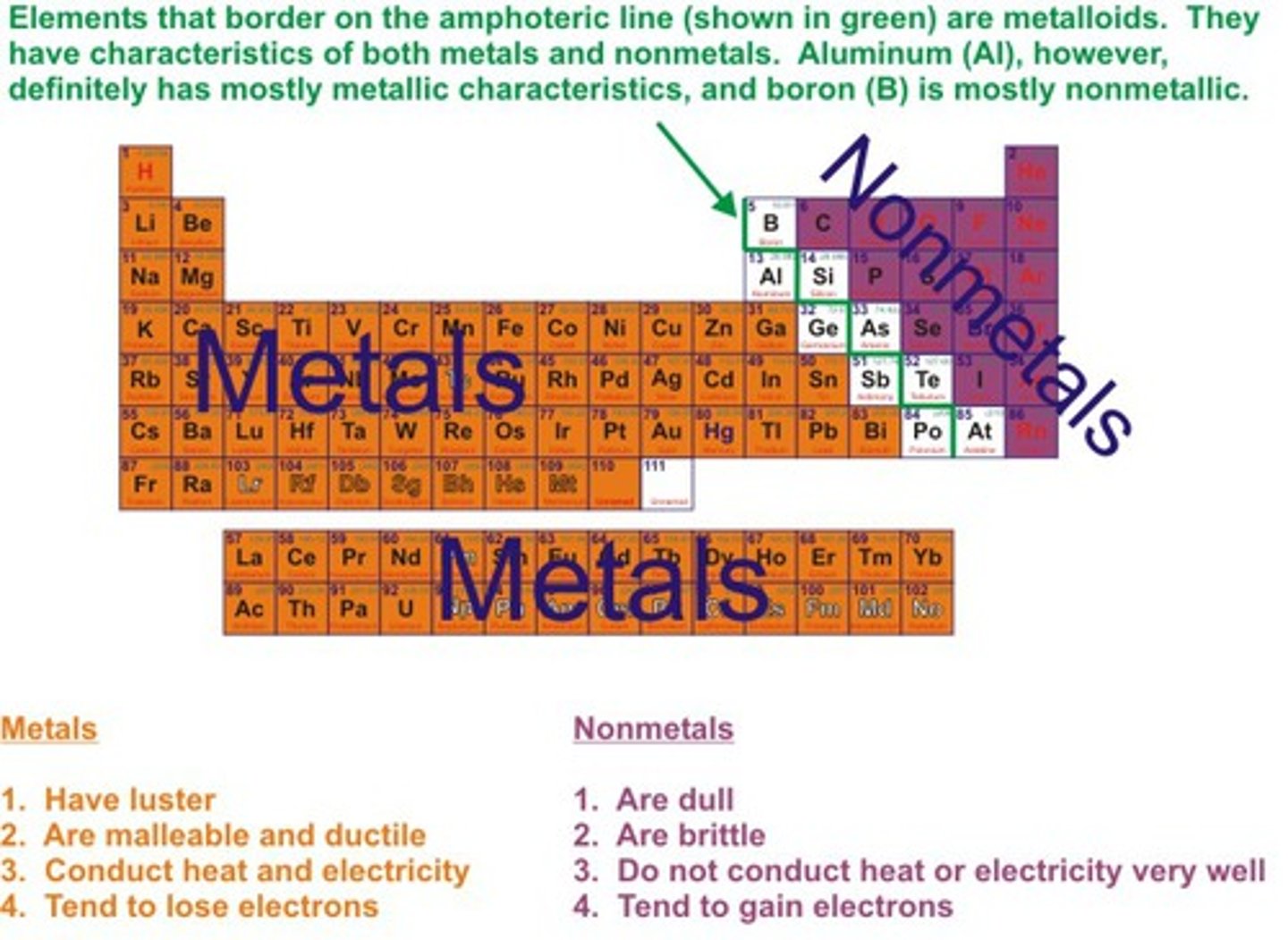
Metals position on periodic table
on the left hand side
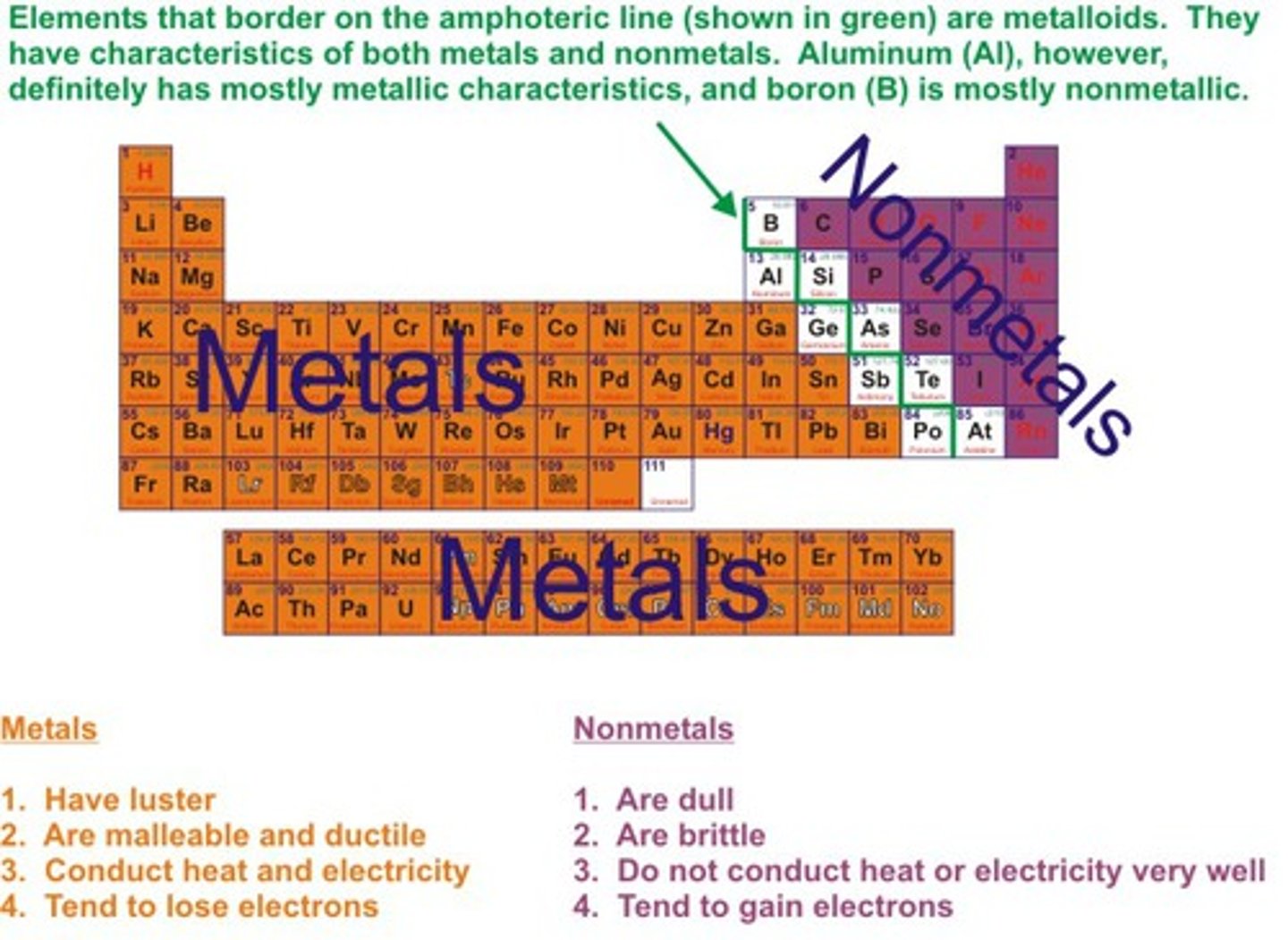
Non-metals position on periodic table
on the right hand side
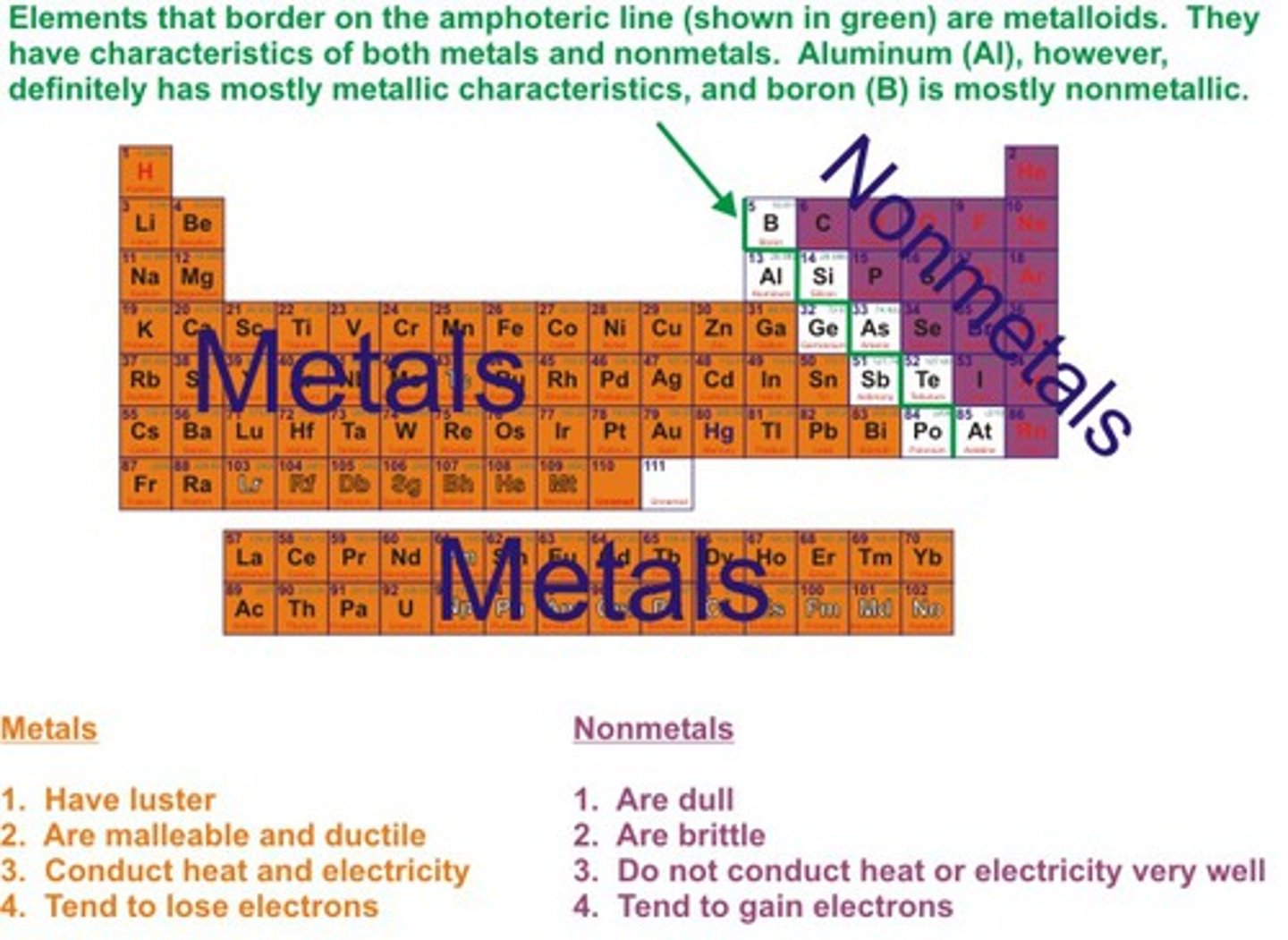
Physical properties of metals
Shiny, malleable, ductile, good conductors of heat and electricity

Physical properties of non-metals
Dull, brittle, insulators of heat and electricty, liquids and gases at room temperature

Malleable
easy to shape or bend

Ductile
Easily stretched into a wire

Good conductor of heat
Allows heat energy to travel through with ease
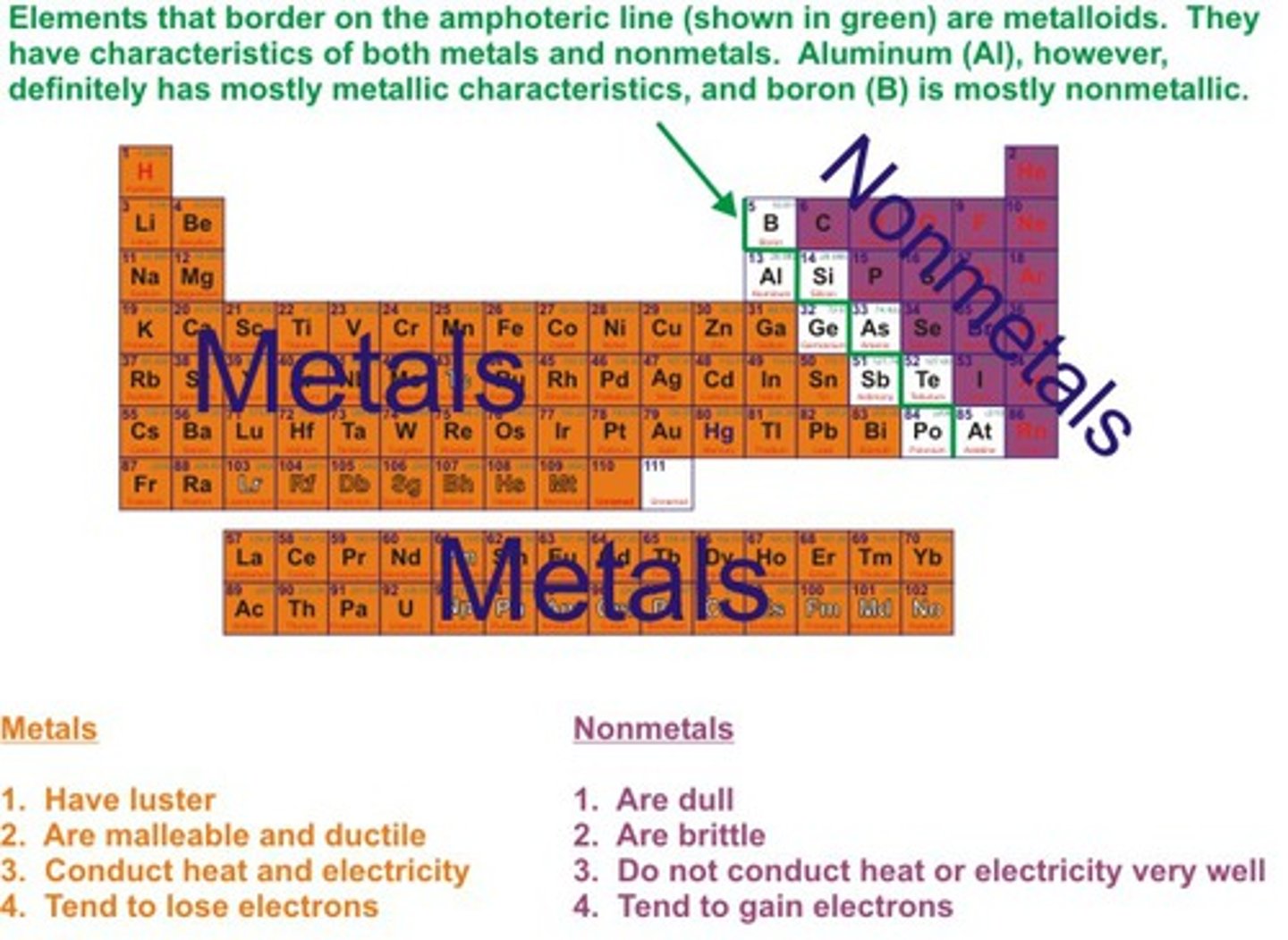
Good conductor of electricity
Allows electrical current to flow through with ease
Brittle
Easily broken

Insulator of heat
a material that does not allow heat to pass through easily

Insulator of electricity
a material that does not allow electric current to pass through easily
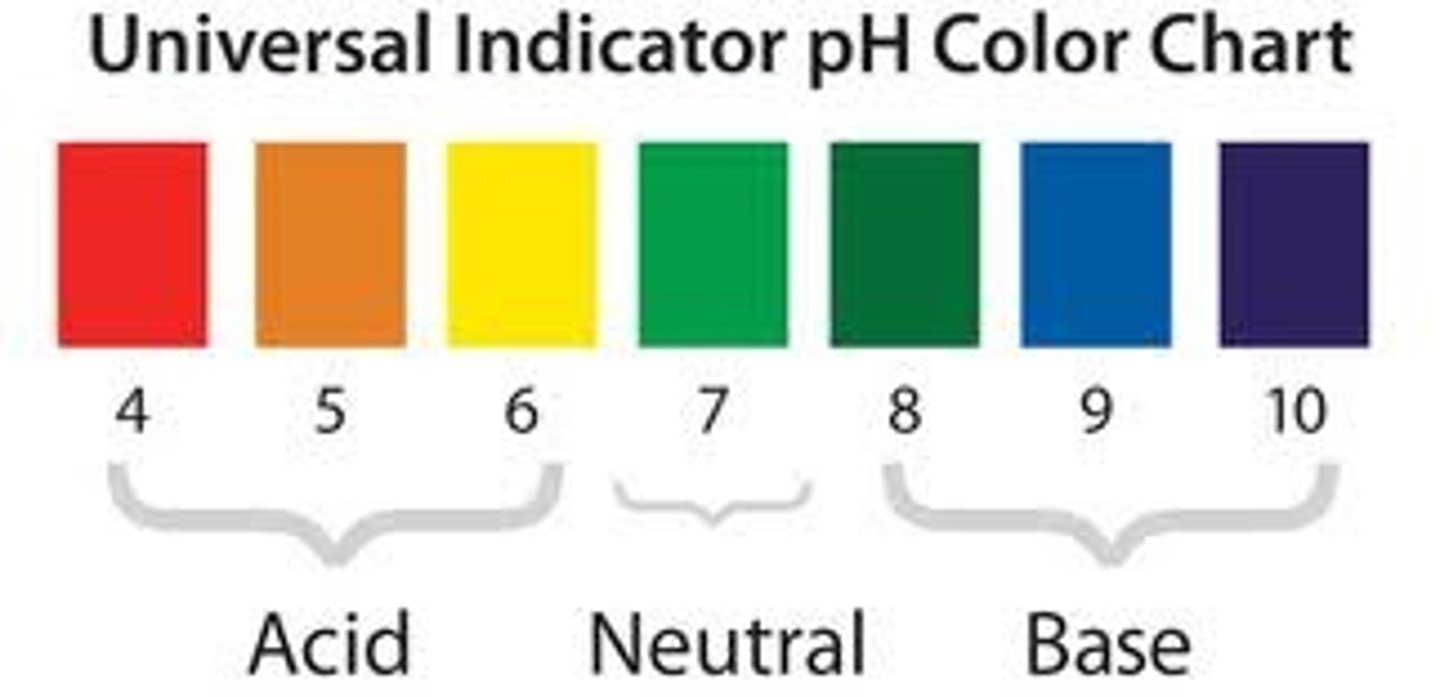
pH of metal oxides
acidic
pH of non-metal oxides
basic