Synthetic transformations
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
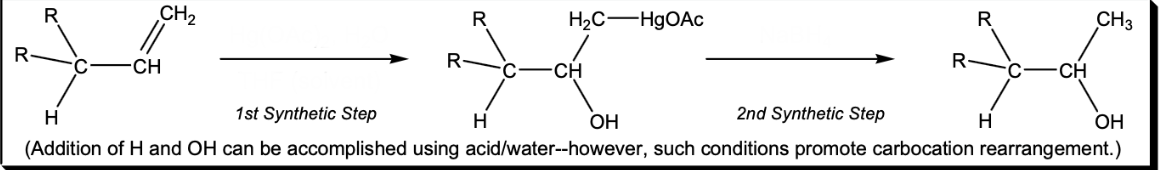
Addition of H and OH without rearrangement
1) Hg(OAc)2, H2O, THF 2) NaBH4
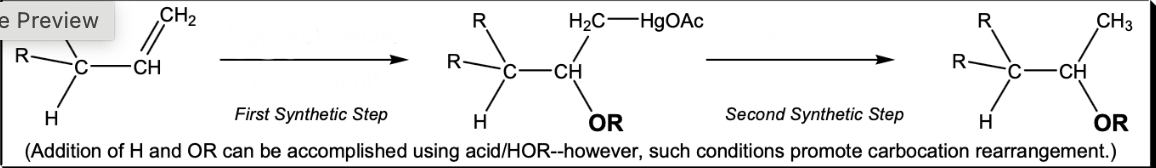
Addition of H and OR without rearrangement
1) Hg(OAc)2, HOR, THF 2) NaBH4
Anti-Markovnikov Addition of H and Br
HBr, peroxides, heat or light
Markovnikov addition of H and X (X = Br, Cl, I)
HX, cold, dark, no peroxides
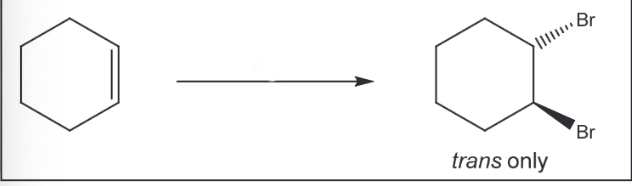
Addition of Br2 to an Alkene
Br2
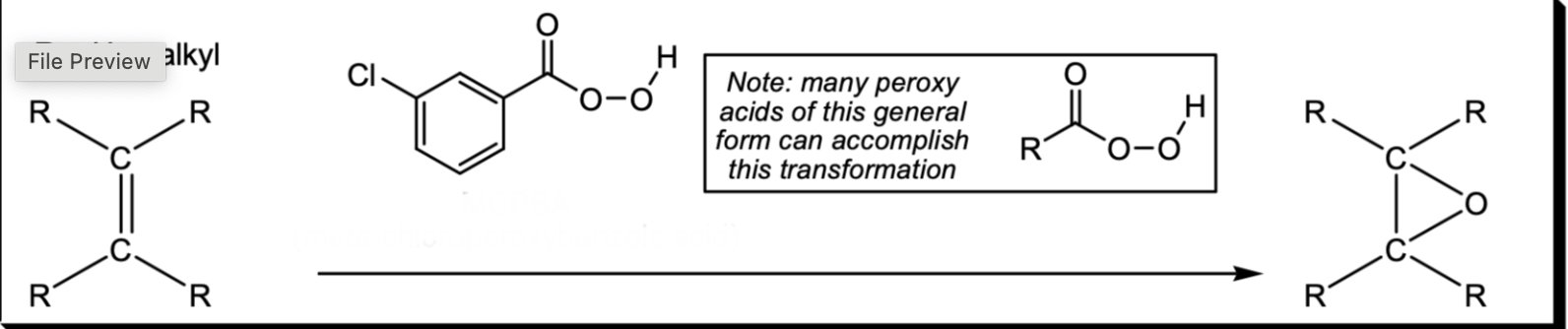
Oxidation of an Alkene to an Epoxide
MCPBA
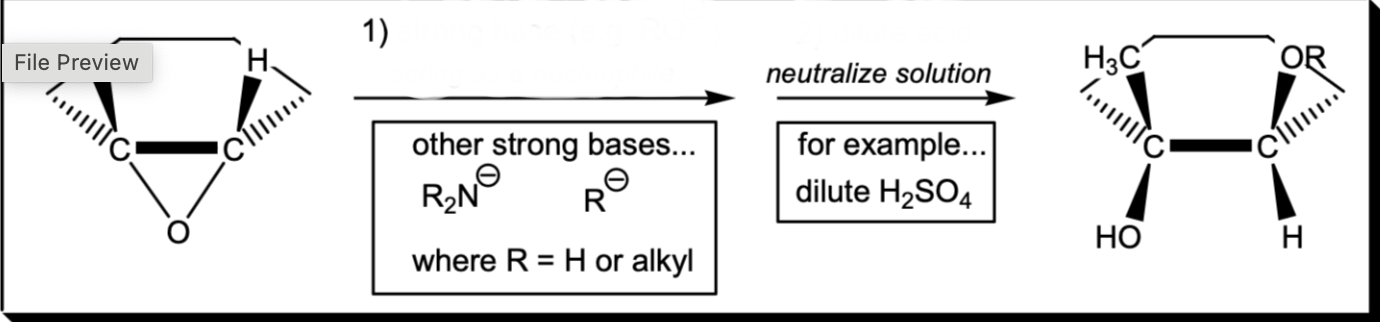
Anti-markovnikov epoxide ring opening (Basic Conditions)
1) Strong Base (HO-) 2) dilute acid (H2SO4)
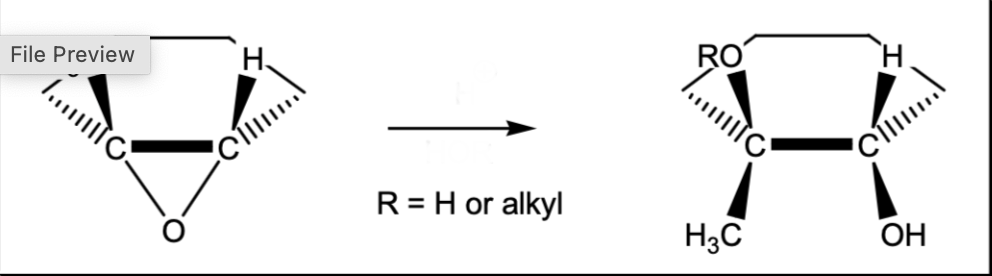
Markovnikov epoxide ring opening (acidic conditions)
H+, HOR
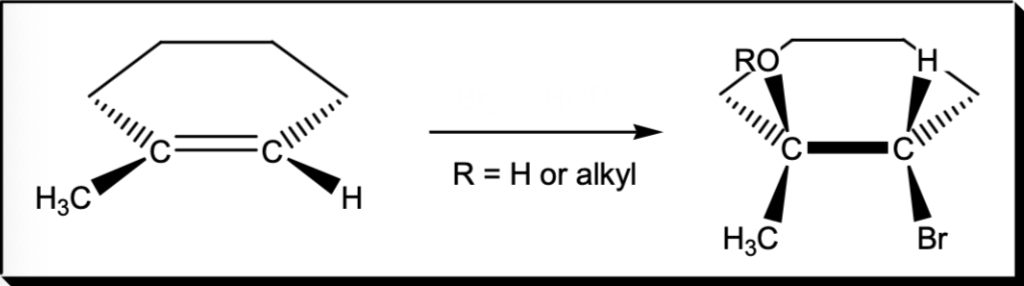
Markovnikov addition of Br and OR to an Alkene
Br2, HOR
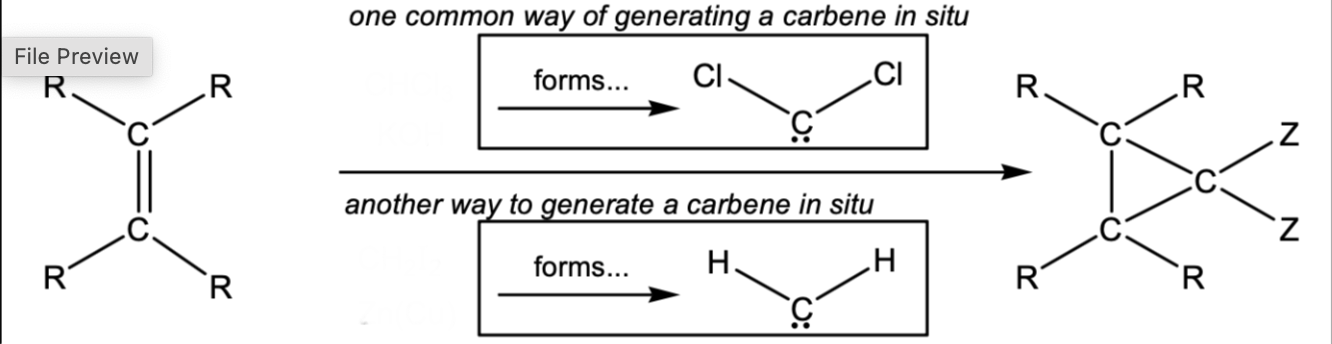
Cyclopropane ring from an alkene
CHCl3, KOH or CH2I2, Zn(Cu)
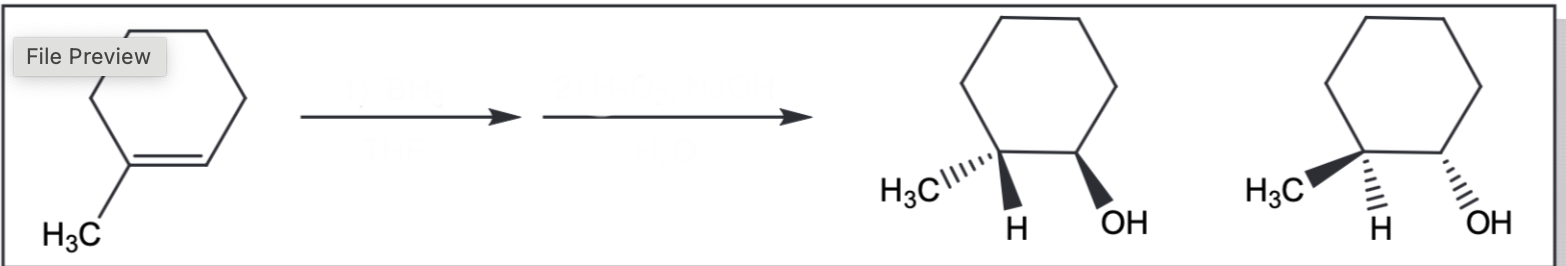
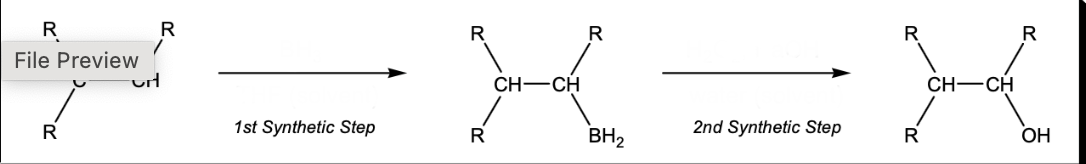
Hydroboration-oxidation
1) BH3, THF 2)H2O2, NaOH. H2O
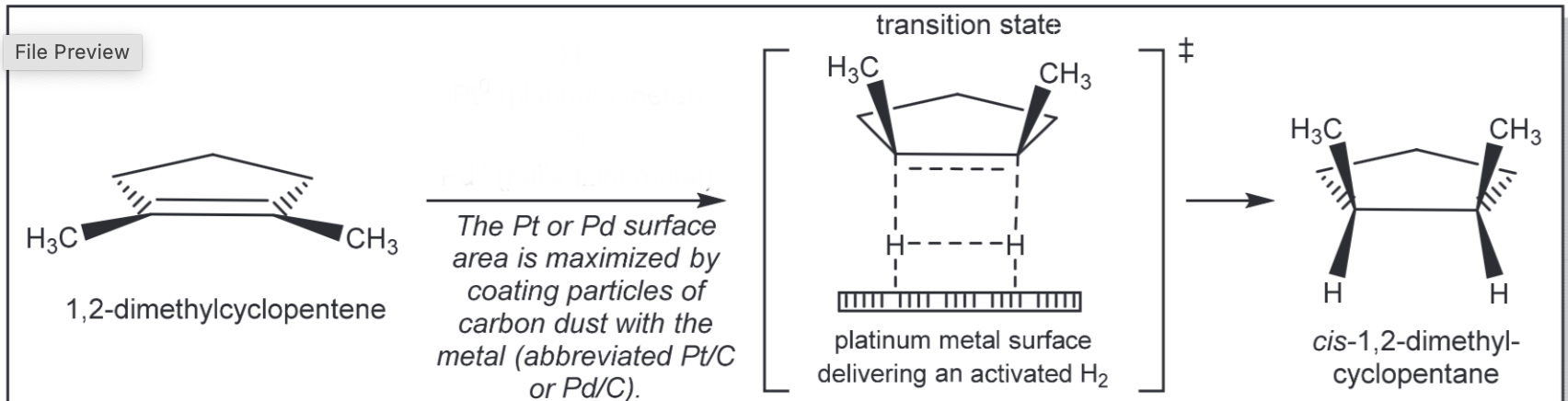
Catalytic hydrogenation
H2, Pt0, Pd0
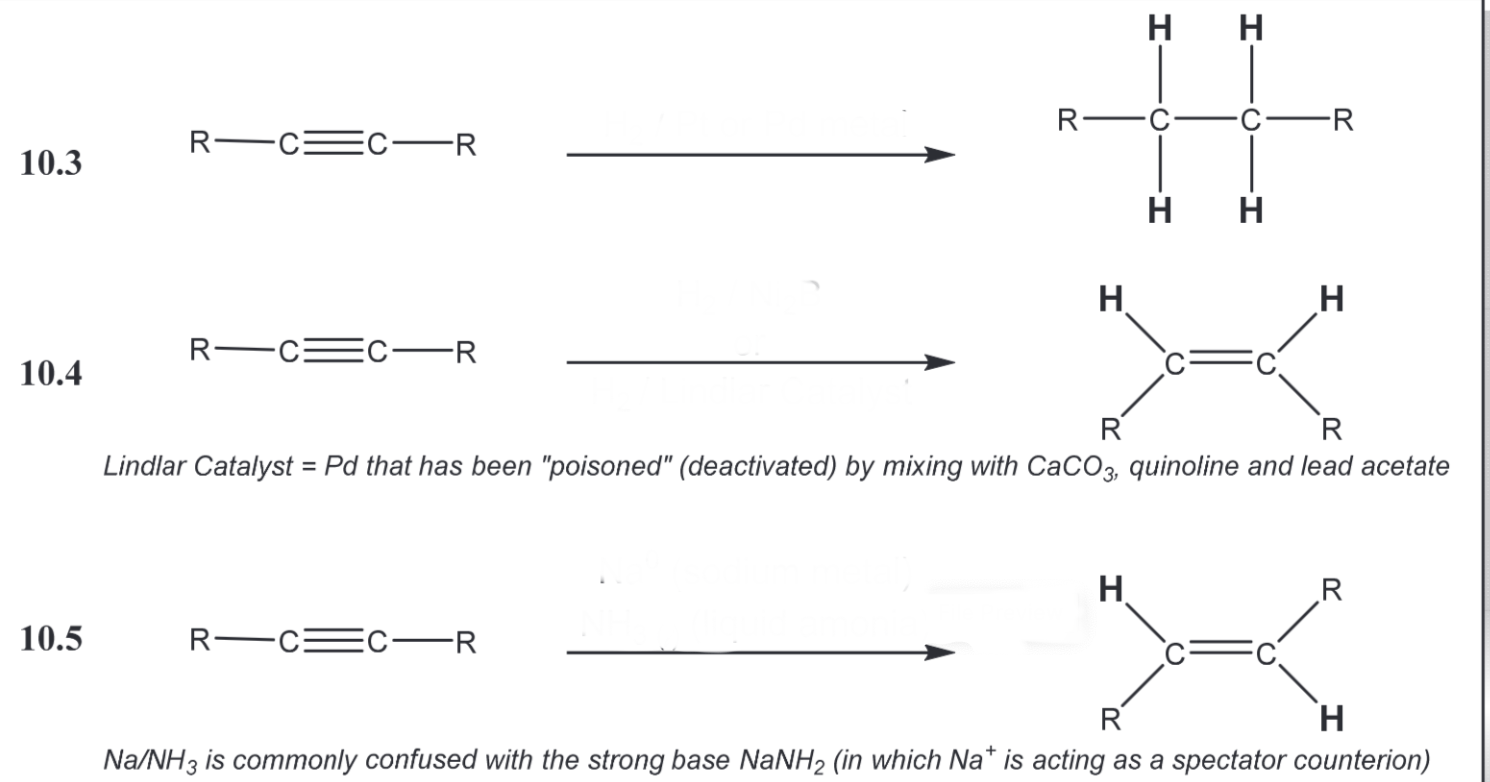
Stereoselective alkyne reductions (look at photo)
1) H2, Pt or Pd 2) H2, Ni2B or H2, Lindar Catalyst 3) Na0, NH3
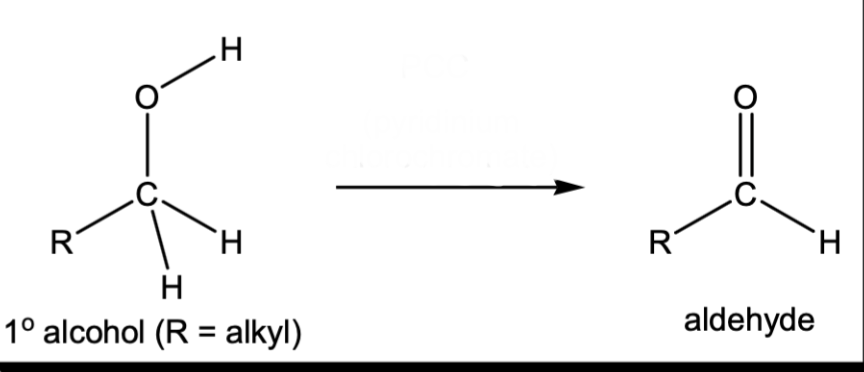
Oxidation of 1 degree alcohol to an aldehyde
PCC
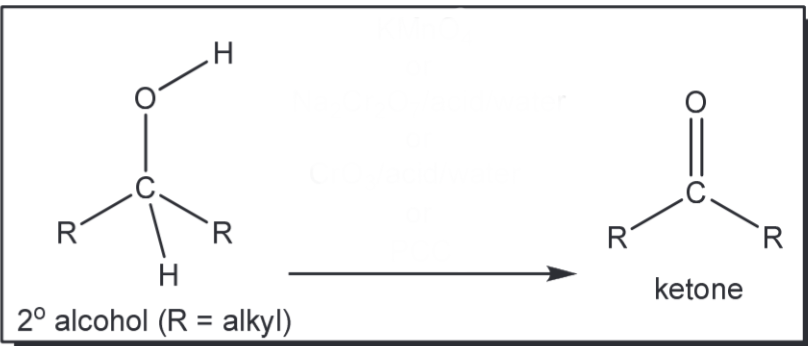
Oxidation of 2 degree alcohol to a ketone
KMnO4 or Na2Cr2O7, acid, water or CrO3, acid, water or PCC
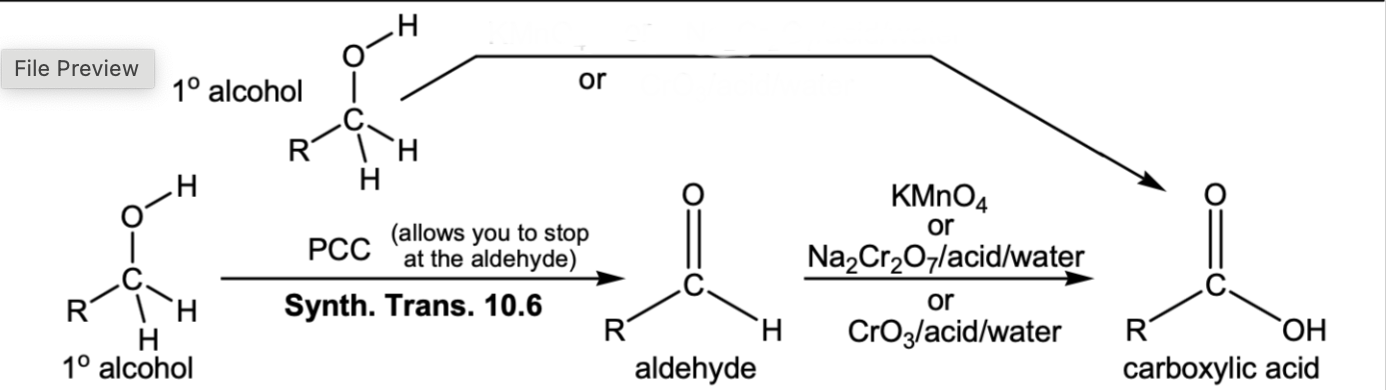
Oxidation of 1 degree alcohol or aldehyde to a carboxylic acid
KMnO4 or Na2Cr2O7, acid, water or CrO3, acid, water
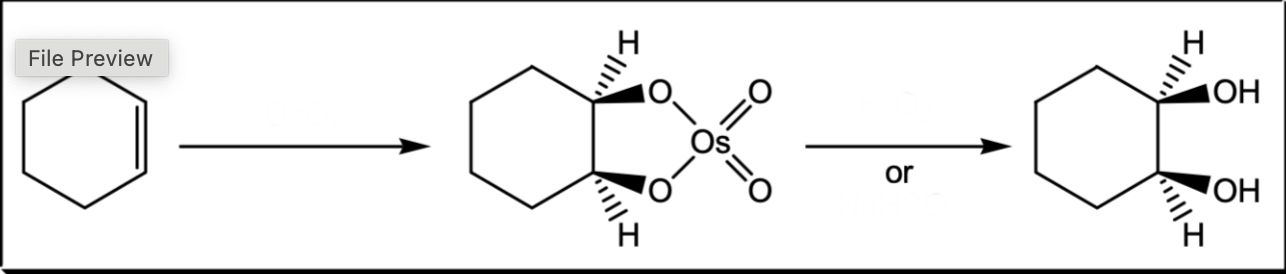
Syn addition of two OH groups to a pi bond
1) OsO4 2) H2O2 or NaHSO3
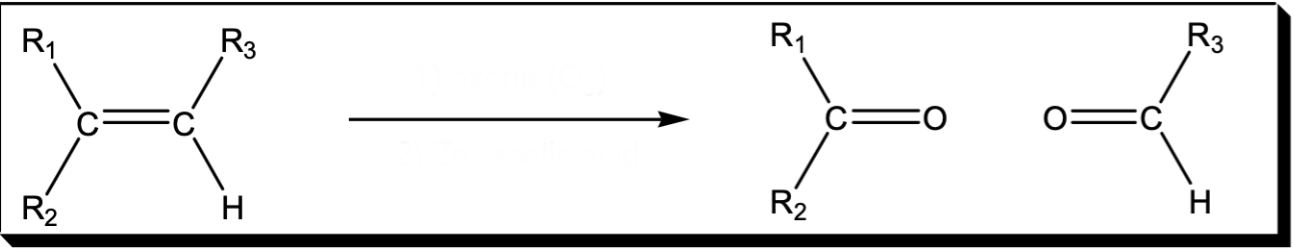
Cleavage of a C=C double bond
1) O3 2) Zn, acetic acid
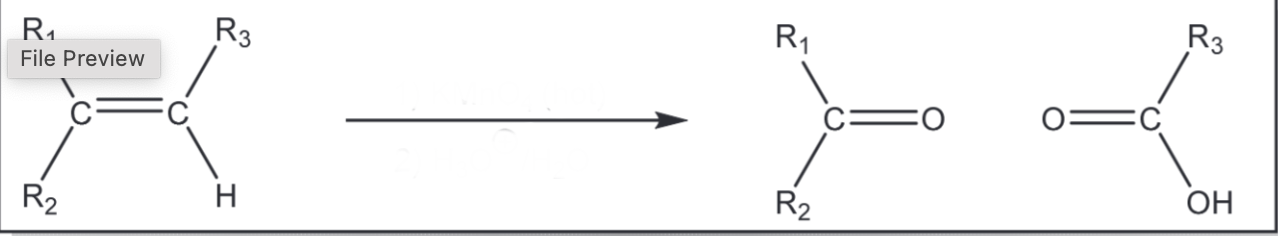
Cleavage of a C=C double bond
1)KMnO4 (hot) 2) H3O+, H2O
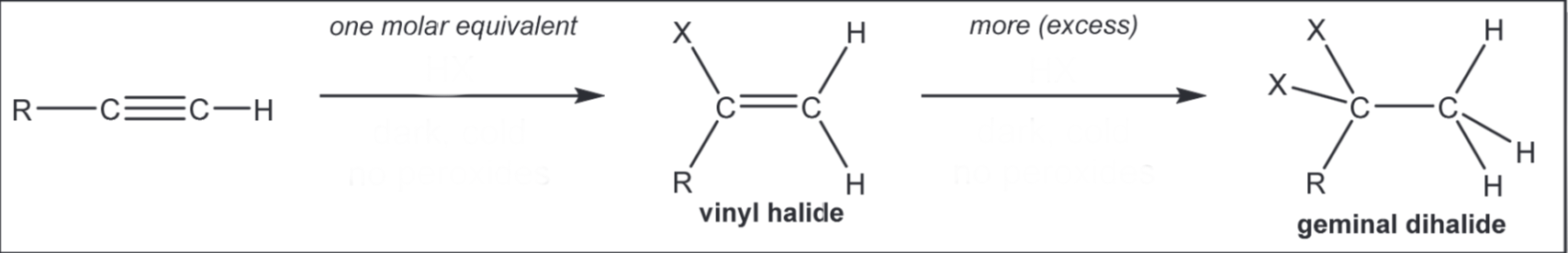
Markovnikov addition of H and X (X = Cl, Br, I)
1) HX, dark, cold, no peroxides 2) HX, dark, cold, no peroxides
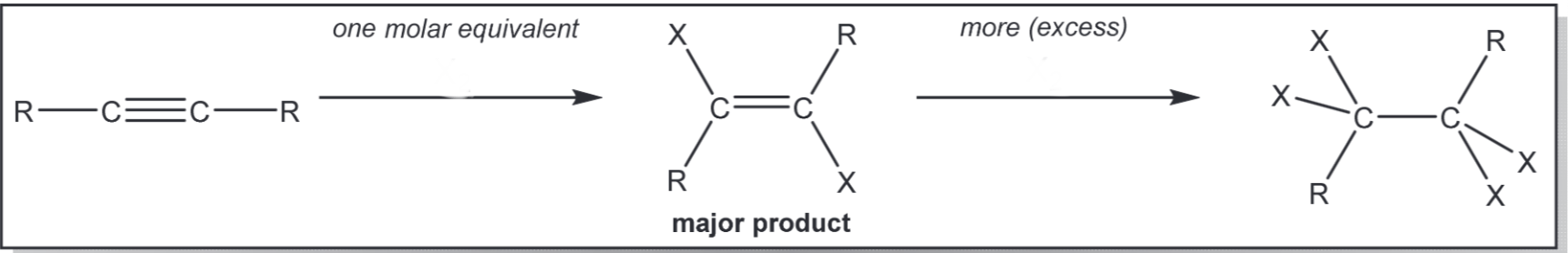
Addition of X2 (X = Cl or Br)
1) X2 2) X2

Markov additions of H2O to an alkyne
H2O, H2SO4, HgSO4
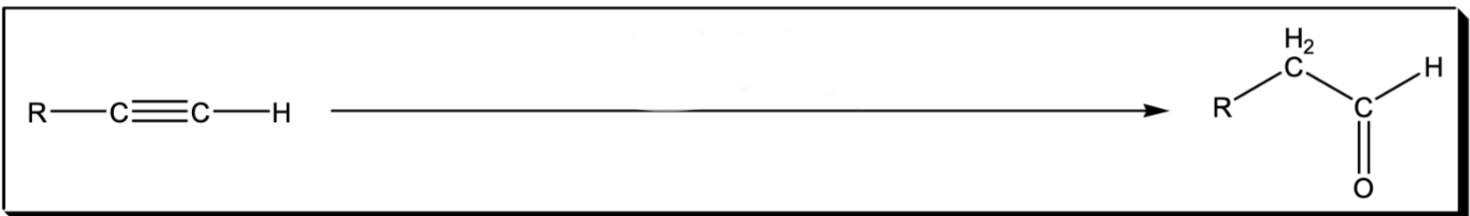
Anti markov additions of H2O to an alkyne
1) BH3, THF 2) H2O2, NaOH, H2O
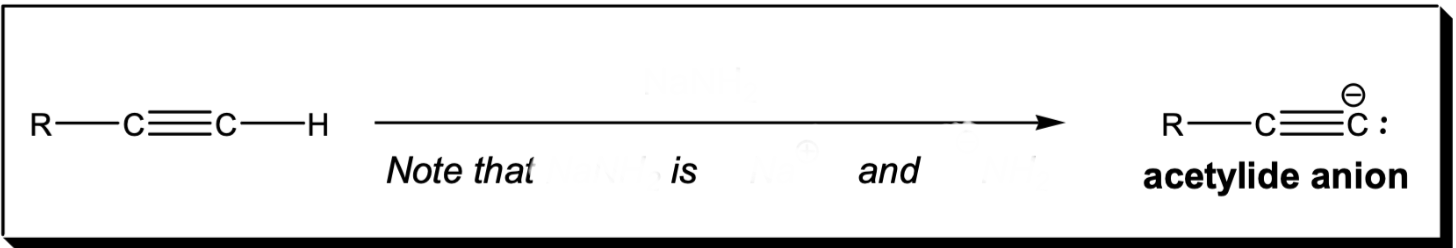
Deprotonation of an alkyne
NaNH2
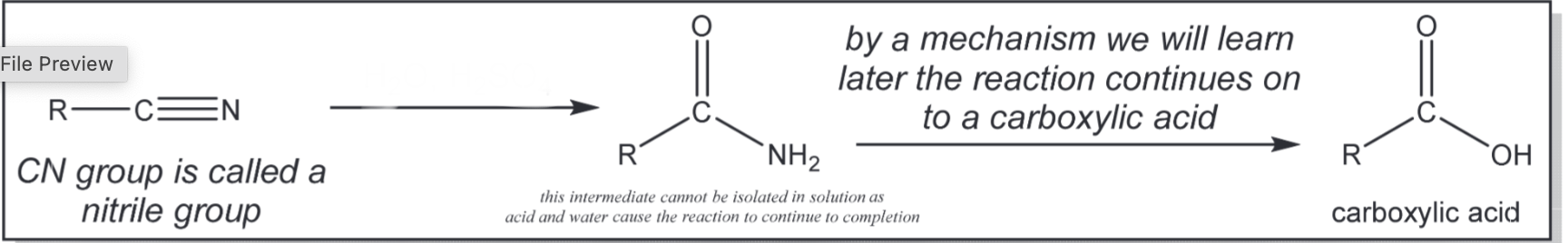
Nitrile hydrolysis
H2O, H2SO4
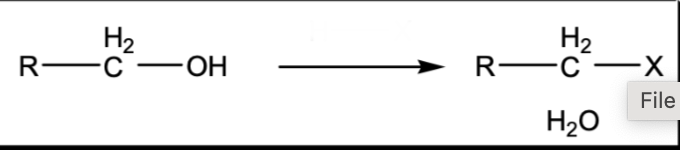
Halogenation of an alcohol
HX
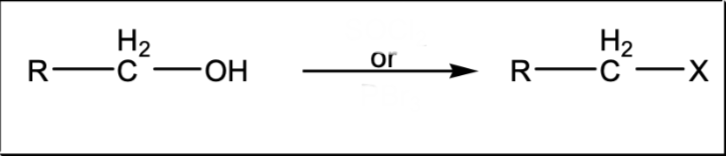
Halogenation of an alcohol
SOCl2 or PBr3