ACS CHM 131 Final Quick Facts
1/57
Earn XP
Description and Tags
Last minute studying for my Gen CHM 1 Final Exam LOL
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
definition of isotopes
An isotope refers to atoms with the same number of protons but different numbers of neutrons. Isotopes can have different atomic masses and may exhibit different nuclear properties, but they belong to the same element.
definition of ion
Atoms with the same number of protons but different numbers of neutrons.
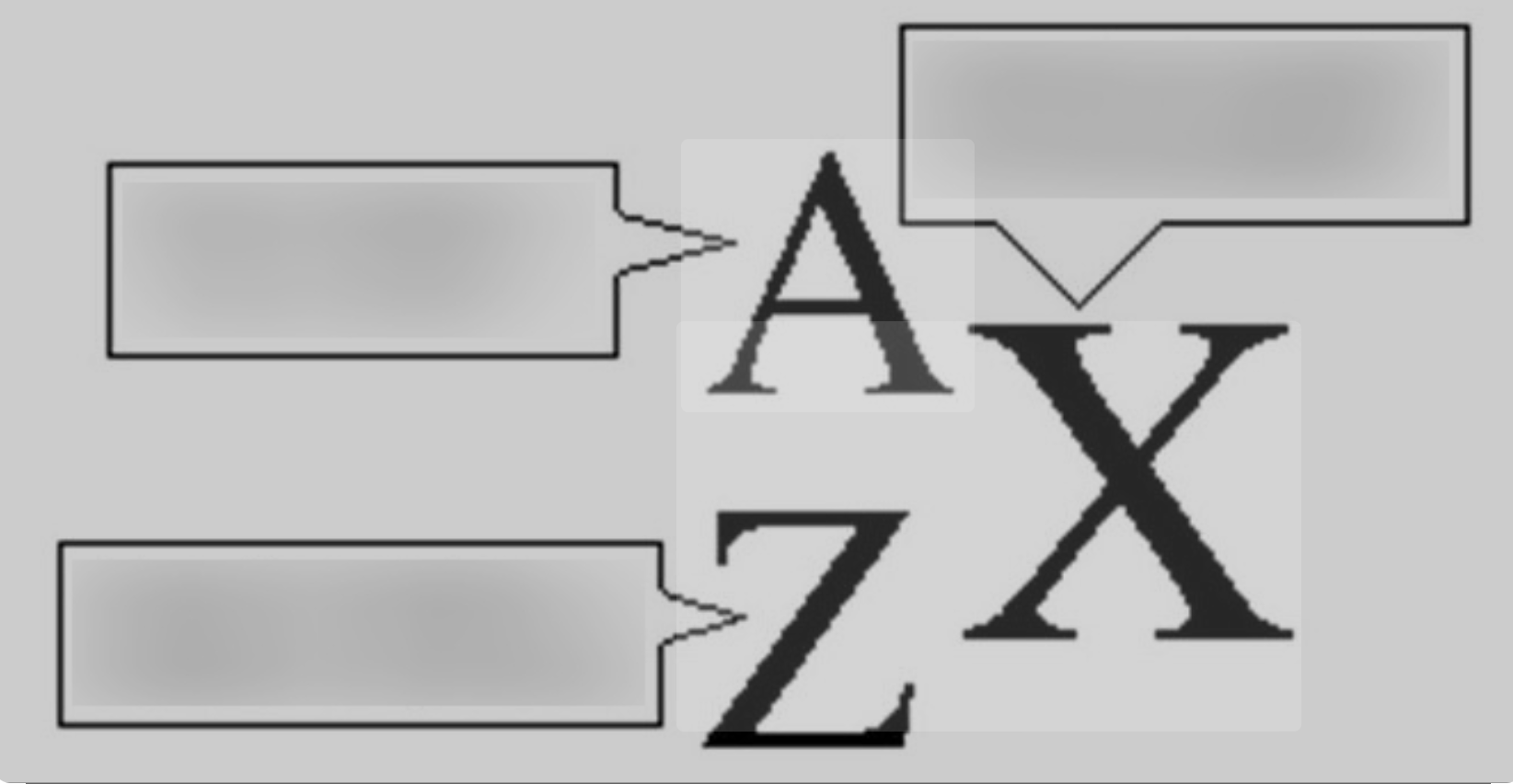
X, A, Z
X = element symbol
A = mass number (protons + neutrons)
Z = atomic number (number of protons)
The relationship between the charge of the ion and the number of electrons and protons
Ion charge equals number of protons minus the number of electrons. A positive charge indicates fewer electrons than protons, while a negative charge indicates more electrons.
definition relative abundance
The percentage of a specific isotope present in a naturally occurring sample of an element.
How to calculate a weighted average:
Weighted average=∑(value×weight)
Example:
Isotope A: 10.0 amu and 60% abundant
Isotope B: 11.0 amu and 40% abundant
Weighted average= (10.0 amu × 0.60) + (11.0 amu × 0.40) = 10.4 amu.
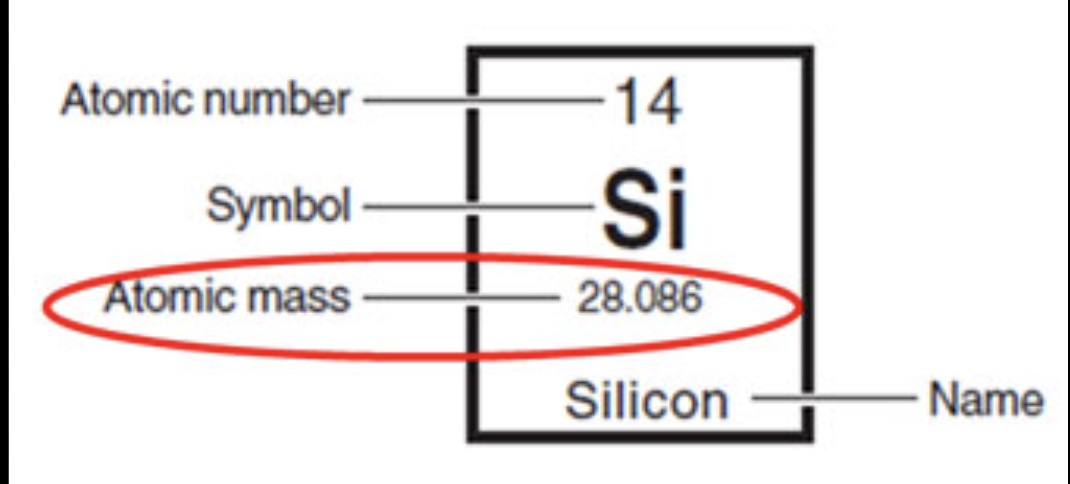
Average atomic mass:
Identifying group number from the periodic table.
Groups are the columns numbered 1 through 18 from left to right.
Group 1 = Alkali metals (e.g., Li, Na)
Group 2 = Alkaline earth metals (e.g., Mg, Ca)
Groups 3–12 = Transition metals
Group 17 = Halogens (e.g., Cl, Br)
Group 18 = Noble gases (e.g., Ne, Ar)
Diatomic molecular elements:
H2, N2, O2, F2, Cl2, Br2, I2
Identifying type of element using periodic table
Rydberg formula
Describes the wavelengths of spectral lines in hydrogen and other hydrogen-like atoms, allowing calculation of energy levels. 1/λ = RH (1/n²1 - 1/n22). RH= Rydberg constant, represents the highest energy transition (1.097×10-7m)
Calculation of wavelength from an energy.
E = hc/λ. h = Planck’s constant (6.626 × 10-34), c = speed of light (3.00 × 108 m/s) , E is the energy of the photon.
Relationship between the delta H of the transition and the energy of the photon emitted
The energy of the photon emitted during the transition is equal to the change in enthalpy (ΔH). ΔH = Energy of photon.
Difference between absorption and emission
Absorption: occurs when an atom or molecule takes IN energy, promoting electrons to higher energy levels.
Emission: happens when an electron drops to a lower energy level, releasing energy as light.
Relationship between energy and wavelength
Longer wavelength → lower energy
Shorter wavelength → higher energy
Rules for quantum numbers:
Principal quantum number (n): The energy level of an electron and its distance from the nucleus. The higher the value of n, the higher the energy level and the farther the electron is from the nucleus. The energy increases as n increases.
Angular Momentum Quantum Number (l): The shape of the orbital. It is related to the angular momentum of the electron in its orbit.
Magnetic Quantum Number (mₗ): The orientation of the orbital in space relative to the other orbitals.
Spin Quantum Number (mₛ): The direction of the electron’s spin, which can be either up or down.
Quantum number constraints
n ≥ 1
l must be in the range 0 ≤ l ≤ n −1
mℓ must be in the range −l ≤ mℓ ≤ l
ms =+1/2 or ms=−1/2
letter abbreviations for values of the l quantum number
l = 0: s orbital (sharp)
l = 1: p orbital (principal)
l = 2: d orbital (diffuse)
l = 3: f orbital (fundamental)
Valence electrons:
Electrons in the outermost shell of an atom that are involved in chemical bonding. They determine an element's chemical properties and reactivity.
Definition of paramagnetic
A type of magnetism that occurs in materials with unpaired electrons, causing them to be attracted to magnetic fields.
Definition of Zeff
In simple terms, Zeff is the attraction an electron feels from the nucleus after accounting for the repulsion from other electrons. Zeff = Z - S, where Z is the atomic number and S is the shielding constant.
Periodic trend for atomic radius
The periodic trend for atomic radius describes how the size of atoms changes as you move across a period (left to right) or down a group (top to bottom) in the periodic table.
The periodic trends in ionic radii
Cations (positive ions):
Ionic radius decreases across a period.
Ionic radius increases down a group.
Cations are always smaller than their neutral atoms.
Anions (negative ions):
Ionic radius decreases across a period.
Ionic radius increases down a group.
Anions are always larger than their neutral atoms
The definition of ionization energy
The energy required to remove an electron from an atom or ion in its gaseous state. It increases across a period and decreases down a group.
How to determine molar mass:
Sum up the molar masses (amu) of all the elements in the compound, considering the number of atoms of each element present.
Converting mass to moles
Moles = Mass(g) / Molar mass (g/mol)
How to use Avogrado’s number
This number is used to convert between the number of moles of a substance and the number of individual particles. 6.022 × 1023.
How to determine mole ratios from molecular formulas
For a compound:
Look at the molecular formula.
Identify the ratio of atoms of each element in the formula.
Simplify the ratio if possible.
For a chemical reaction:
Write or refer to the balanced chemical equation.
Use the coefficients to determine the mole ratio between reactants and products.
Apply the mole ratio to convert between amounts of reactants and products.
How to convert from moles to grams.
Grams = Moles × Molar Mass (g/mol)
How to determine limiting reactant and theoretical yield
Write the balanced chemical equation for the reaction.
Convert the mass of each reactant to moles.
Determine the limiting reactant by comparing the mole ratios of the reactants.
Calculate the theoretical yield based on the limiting reactant using the stoichiometric ratios from the balanced equation.
definition of an electrolyte:
A substance that dissolves in water to produce ions and is capable of conducting electricity.
Classification of strong vs. weak electrolytes.
Strong Electrolytes: Completely dissociate in water, producing a high concentration of ions. Examples include salts like NaCl, strong acids like HCl, and strong bases like NaOH.
Weak Electrolytes: Only partially dissociate in water, producing a lower concentration of ions. Examples include weak acids like acetic acid (CH₃COOH) and weak bases like ammonia (NH₃).
Definition of molar concentration
Molar concentration (also called molarity) is a way to express the concentration of a solute in a solution. It refers to the number of moles of solute present in one liter of solution.
M= moles of solute/liters of solution
Process of Dilution
The dilution equation relates the initial and final concentrations and volumes of the solution:
C1V1=C2V2
Definition of oxidation number:
The hypothetical charge it would have if the bonding were purely ionic, assuming that the more electronegative element in the bond takes all the shared electrons.
Rules for assigning oxidation numbers
Rules that dictate how to assign oxidation states to elements in compounds, guiding how to determine the hypothetical charge of atoms.
For any atom in its elemental form (e.g., O₂, N₂, H₂, Cl₂), the oxidation number is always 0
Oxidation number of a monatomic ion is equal to its charge
The sum of oxidation numbers in a neutral compound is 0
The sum of oxidation numbers in a polyatomic ion is equal to the charge of the ion:
Definition of oxidation and reduction
Oxidation: The process in which an atom, ion, or molecule loses electrons. As a result, its oxidation state (or oxidation number) increases.
Reduction: The process in which an atom, ion, or molecule gains electrons. As a result, its oxidation state (or oxidation number) decreases.
First Law of thermodynamics:
Energy cannot be created or destroyed, only transformed.
Sign conventions for heat and work
Heat added to the system: Positive (q>0)
When the system absorbs heat from the surroundings, it gains energy.
Heat removed from the system: Negative (q<0)
When the system releases heat to the surroundings, it loses energy.
Work done by the system on the surroundings: Positive (W>0)
When the system does work on the surroundings (e.g., a gas expands and pushes against a piston), the system loses energy.
Work done on the system by the surroundings: Negative (W<0)
When work is done on the system (e.g., a piston compresses the gas), the system gains energy.
Definition of Specific Heat
The amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius (°C) or one Kelvin (K).
Specific heat capacity equation:
q = m⋅c⋅ΔT
Concept of energy per mole of a reaction.
The amount of energy that is either absorbed or released when one mole of reactants undergoes a chemical reaction.
For exothermic reactions, energy is released and the energy per mole is negative.
For endothermic reactions, energy is absorbed and the energy per mole is positive.
Principles of bomb calorimetry
A method used to measure the heat of combustion of a substance in a controlled environment.
Definition of the enthalpy of formation
The enthalpy change when 1 mole of a compound is formed from its constituent elements in their most stable form at standard conditions.
The elements must be in their standard states (e.g., O₂ as a gas, C as graphite, H₂ as a gas).
The product must be exactly 1 mole of the compound.
The standard enthalpy of formation of any element in its standard state is defined as 0 kJ/mol.
Rules for manipulating enthalpy values
If you reverse a chemical equation, change the sign of the enthalpy (ΔH)
If you multiply a balanced equation by a coefficient, multiply the ΔH by the same factor.
If you add two or more chemical equations, add their ΔH values to get the overall enthalpy change.
The ΔHf∘ of any element in its standard state is 0.
If a reaction can be expressed as a sum of multiple steps, the total enthalpy change is the sum of the enthalpy changes of the steps.
Hess’s Law
States that the total enthalpy change for a reaction is the same regardless of the pathway taken, as long as the initial and final conditions are the same.
Definition of lattice energy:
Lattice energy is the enthalpy change that occurs when gaseous cations and anions combine to form one mole of an ionic solid.
Definition of bond types
The different ways atoms can chemically combine by sharing or transferring electrons to form molecules or compounds.
Ionic Bond: A bond formed when electrons are transferred from one atom (usually a metal) to another (usually a nonmetal).
Covalent Bond: A bond formed when two nonmetal atoms share one or more pairs of electrons
Metallic Bond: A bond formed between metal atoms, where electrons are delocalized and free to move throughout the structure.
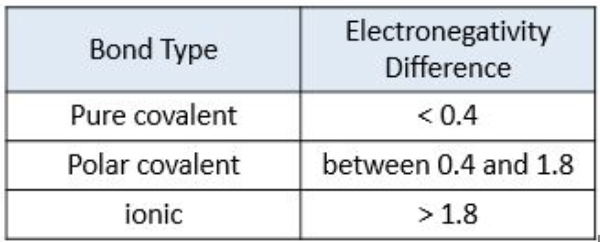
Electronegativity to determine bond type
A measure of an atom's ability to attract shared electrons in a chemical bond.
Definition and how to determine formal charge:
Formal charge: the hypothetical charge an atom would have if all bonding electrons were shared equally between atoms, regardless of their electronegativity.
Formal Charge (FC)=Valence Electrons−Nonbonding Electrons−(1/2×Bonding Electrons)
Draw a valid Lewis structure of the molecule.
For each atom:
Count the number of valence electrons it normally has (periodic table).
Count the number of lone pair electrons on the atom.
Count the number of bonding electrons it shares (double for shared bonds).
Plug the values into the formal charge formula.
Definition of resonance structures
The different ways to represent a molecule where electrons can be distributed over multiple structures without changing the overall molecule.
Covalent bonds:
Chemical bond that involves the sharing of electron pairs between atoms, allowing them to achieve a full outer shell and stability.
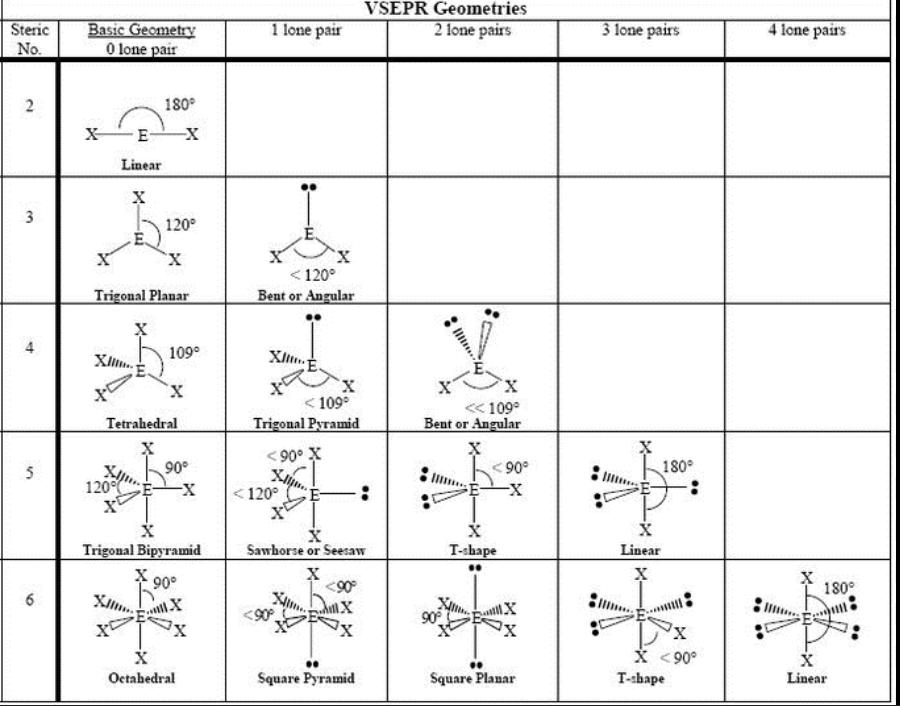
Molecular Geometry
Refers to the three-dimensional shape of a molecule formed by its atoms, determined by the arrangement of bonding pairs and lone pairs of electrons around the central atom.
Draw the Lewis structure of the molecule.
Count the electron domains (bonding and lone pairs) around the central atom.
Use VSEPR theory to predict the arrangement.
Identify the geometry based on how atoms (not lone pairs) are arranged.
Valence bond theory:
Atomic orbitals overlap to form covalent bonds, highlighting the role of electron pairs in bonding.
Molecular shape and corresponding bond angles
the 3D arrangement of atoms around a central atom, and it's primarily determined by the number of bonding and lone pairs of electrons. This is based on VSEPR theory (Valence Shell Electron Pair Repulsion), which assumes electron pairs repel each other and want to be as far apart as possible.
Molecular polarity:
The distribution of electric charge in a molecule, which determines whether it has a positive or negative dipole moment. This property affects interactions between molecules, influencing solubility and boiling points.
Definition of bond order:
The number of chemical bonds between a pair of atoms; calculated as the difference between the number of bonding and antibonding electrons divided by two.