Section A - Structure, Bonding, and Self-Assembly of Biological Structures
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
What is electron configuration?
Number of electrons an atom has and how they are arranged around the nucleus
How are elements arranged in the periodic table?
In a reflection of their electronic configuration and in particular the identity of their valence electrons
Which concepts are required to determine the ground state electron configuration of an atom?
Hund’s Rule = Single occupancy before pairing
Aufbau (‘building up’) Principle = Lowest energy orbitals are occupied first
Pauli (Exclusion) Principle = Only two electrons may occupy each orbital
What does the term degenerate mean in terms of atomic structure and electron configuration?
Identical and indistinguishable in energy
Give an example of a degenerate orbital
The 2s and 2p orbitals for hydrogen have the same energy as each other and are said to be degenerate. Similarly, the 3s, 3p, and 3d orbitals are degenerate
What is the Aufbau ‘building up’ Principle?
Lowest energy orbitals are occupied first
What is the Pauli Principle?
Only 2 electrons occupy each orbital
What is the fundamental property of Quantum Mechanics?
Matter has wave-like properties (wave-particle duality)
What is meant by Wave-Particle Duality in terms of electrons?
Electrons are not just a particle and not just a wave
What is a wavefunction (Ѱ)?
A mathematical description of the distribution of electrons in terms of position and time
How is the wavefunction for an electron calculated?
Using the Schrödinger equation
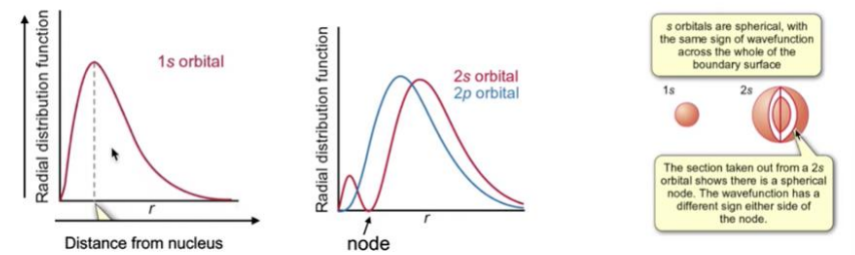
Describe the image
o The further the orbital is from the nucleus, the higher the energy
o Wavefunctions can be used to calculate the radial distribution function of the orbital (how the probability of finding the electron varies with distance from the nucleus)
o For the 1s orbital the electron has the highest probability of being found at the dotted line
o It has a 0 probability of being found at the nucleus, but the chances increase rapidly with r, until the maximum
o The probability of the electron being found a long, long way from the nucleus is very low – tends to zero
o r = probability of electron being found at that distance
o The energy of the orbital depends on the strength of the nuclear charge that the electron feels when it occupies that orbital
o The 1s orbital would shield the 2s orbitals therefore the probability of an electron being further away from the nucleus for the 2s orbitals is higher
What is an implication of describing the electron as a wave?
Heisenberg Uncertainty Principle
What is the Heisenberg Uncertainty Principle?
Position and momentum (energy) of an electron cannot simultaneously be determined
What is Atomic Spectroscopy?
Involves the excitation of electrons between the different orbitals and the determination of which transitions are observed
To know the relative energies of the orbitals and which are occupied
What is an (Atomic) Orbital?
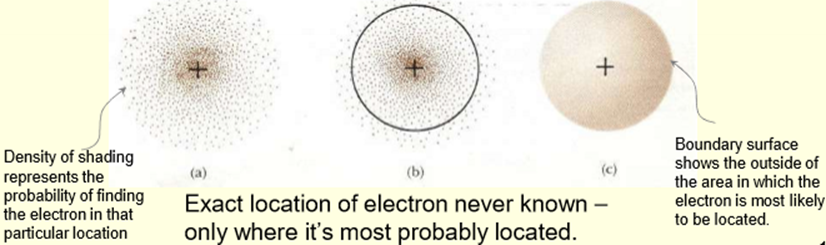
The region of space where an electron (of a specific energy) is most probably located, defined by a wave function
What are Quantum Numbers?
Labels used to describe the orbital shapes and sizes
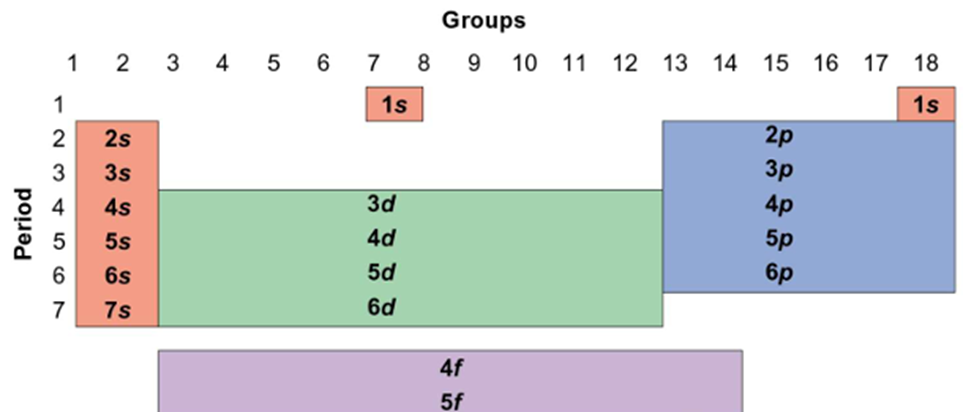
What is the first quantum number?
Principle Quantum Number (n) – shell number
Shells are of ↑ size (or average distance from the nucleus)
PQN tells you how big the orbital is, or how far (on average) an electron occupying it is from the nucleus
n = 1, 2, 3, … (can take any integer, however 1-7 are the most important chemically)
What is the second quantum number?
Angular Momentum Quantum Number (l) – shape of the orbital
Each shell can have more than one subshell, labelled according to their shape: s, p, d...
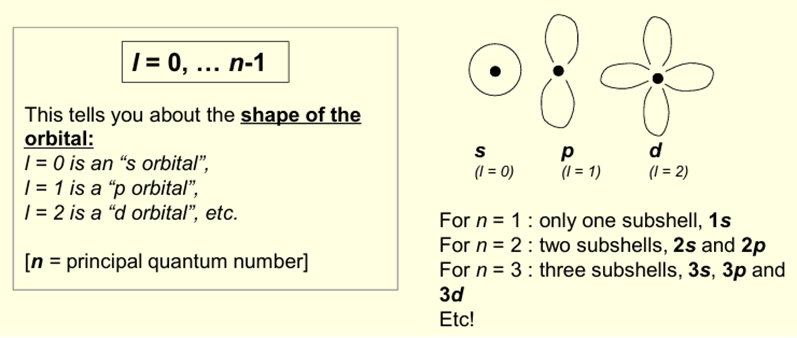
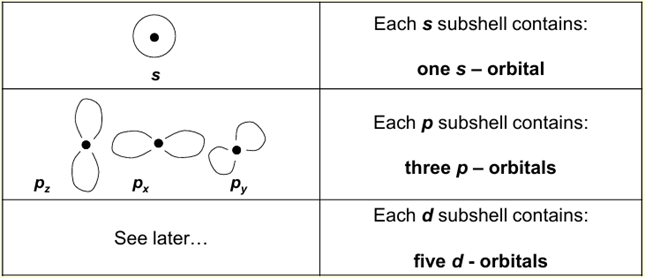
What is the third quantum number?
Magnetic Quantum Number (mI) – orientation of the orbital
- These are the px, py, pz
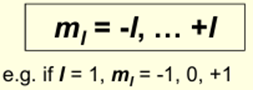
What is the fourth quantum number?
Spin Quantum Number (ms) – electron spin
Need to consider electrons have spin
Can have a principal axis and rotation axis that can spin around, it can spin in 2 direction
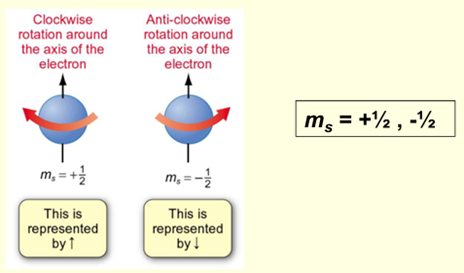
What principle does the fourth quantum number relate to?
Pauli Exclusion Principle
What is the Pauli Exclusion Principle?
No two electrons in an atom can have the same four quantum number
What is the implication of the Pauli Exclusion Principle?
A maximum of two electrons can share each orbital, with the same values of n, l, mI but different spin (ms = +1/2 and -1/2)
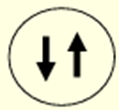
What is the Hund’s Rule of Maximum Multiplicity?
If two or more orbitals have the same energy, then electrons will spread out to occupy the maximum possible number of orbitals, maximising the number of parallel spins of the electrons, minimizing repulsion and lowers the energy of the atom – i.e. single occupancy before pairing
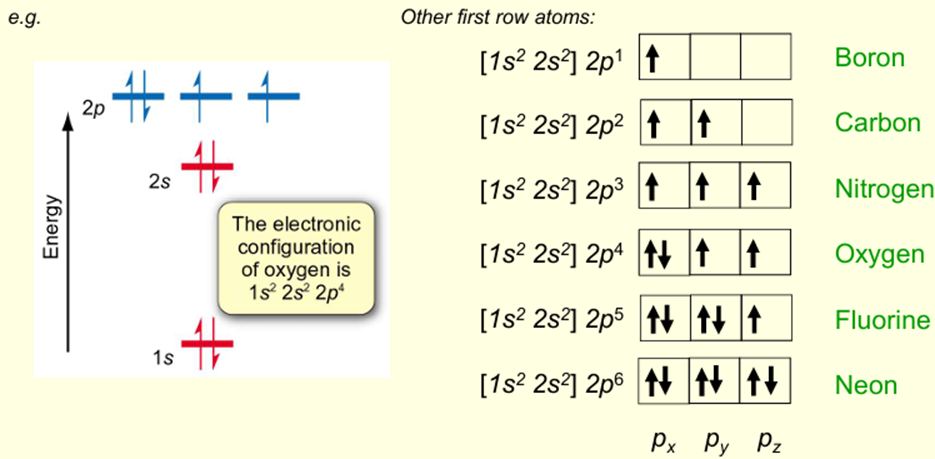
If electrons occupy separate orbitals, then they occupy different regions of space so there is less electrostatic repulsion – lower energy arrangement
Spin Correlation = Electrons have lower energies if their spins are parallel because parallel spins will stay further away from each other so repulsion is reduced
What is Spin Correlation?
Electrons have lower energies if their spins are parallel because parallel spins will stay further away from each other so repulsion is reduced
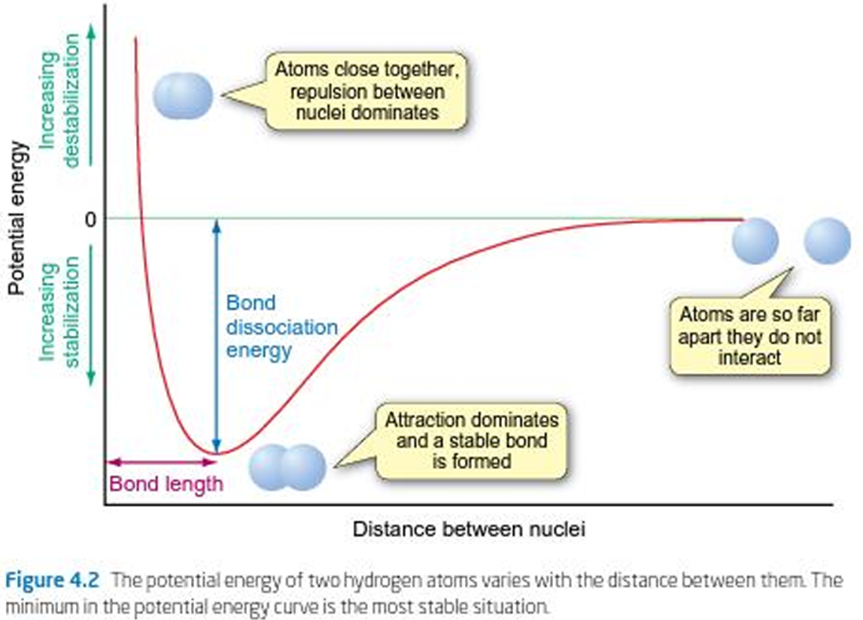
Describe the graph in terms of Covalent Bonding
When 2 atoms move closer to each other, the potential energy of the atoms decreases
Potential energy between 2 atoms varies with the distance between their nuclei
A pair of atoms are considered to have a chemical bond between them when they lie in the ‘energy well’, i.e. at lower energy than the individual atoms
What is the Bond Length (in a diatomic molecule)?
The mean distance between the centres of the two atoms
What is Bond Dissociation Enthalpy?
A measure of the bond strength, defined as the standard enthalpy change of the reaction, at a specified temperature, in which the bond is broken
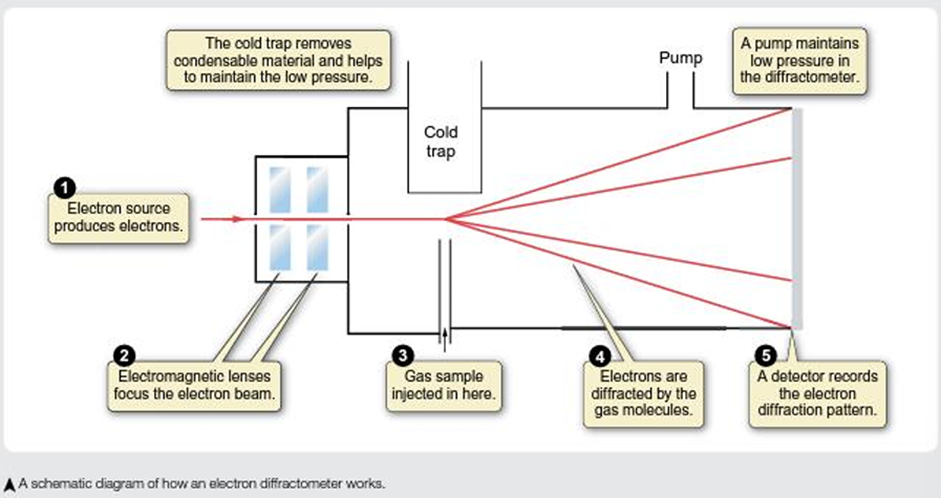
How can we measure bond lengths?
Measure using diffraction techniques, for molecules in the gas phase = Electron diffraction
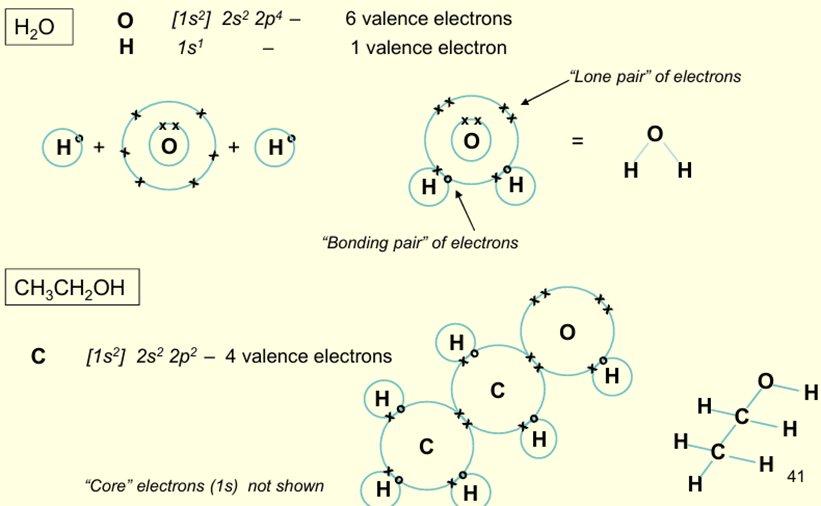
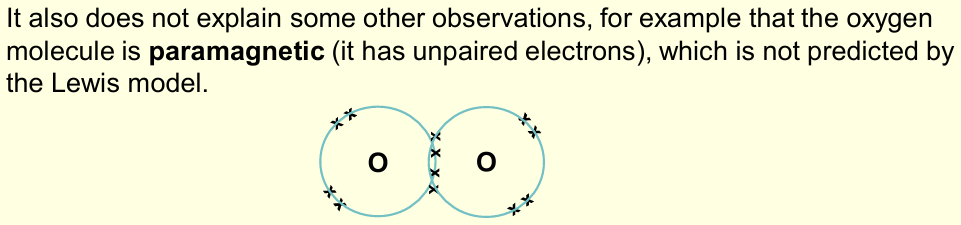
What is meant by the Lewis Model?
Covalent bonding occurs when valence electrons are shared between 2 atoms
When is the maximum stability achieved?
When each atom has a ‘filled valence shell’. In this case each H has a full 1s orbital
What is the meant by the Octet Rule?
Each atom acquires shares in electrons until its valence electrons achieves 8 electrons
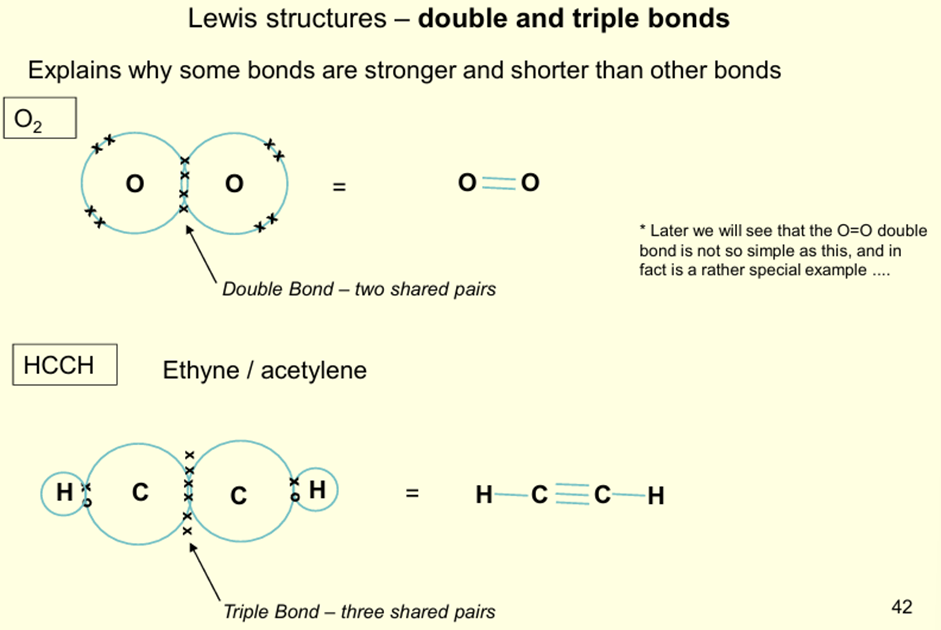
Why are some bonds stronger and shorter than other bonds?
Double Bond - two shared pairs
Triple Bond - three shared pairs (stronger but shorter)
What is meant by the Lewis Model of Ions?
Molecules can accept or lose electrons to give a complete valence shell
Result = -ve / +ve ion
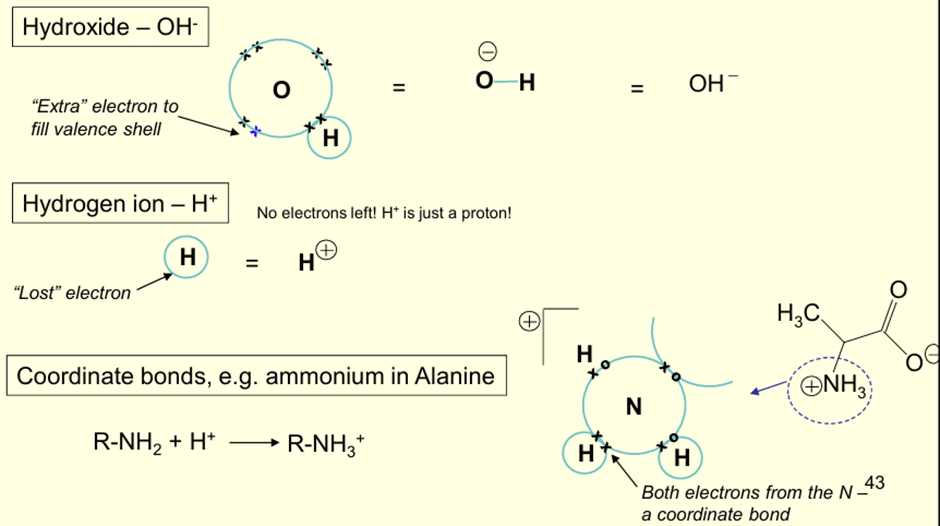
What is meant by Coordinate Bonds?
Both electrons come from one atom to form a covalent bond
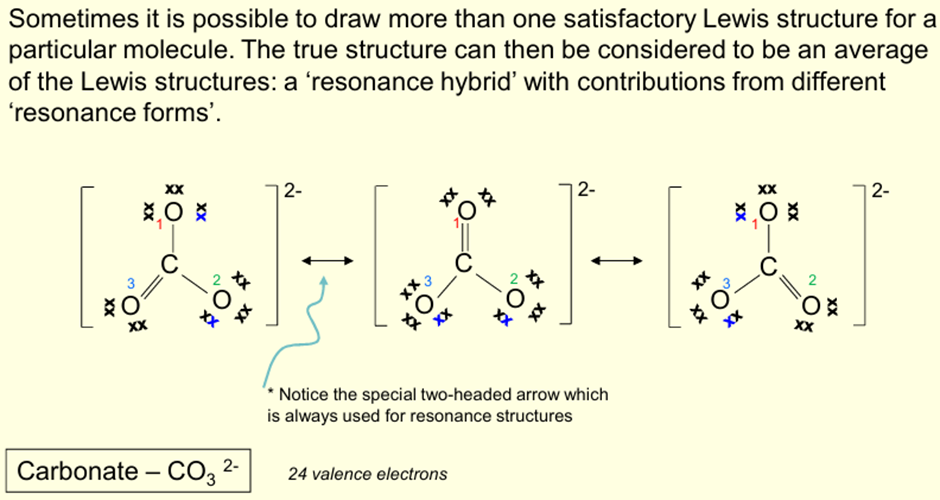
What is meant by Resonance Hybrids?
Possible to draw more than one satisfactory Lewis structure for a particular molecule
What are Hypervalent Compounds?
Compounds that require more than an octet of electrons in order to draw a Lewis structure
What are the basic assumptions of the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory)?
Electrons in bonds and lone pairs around an atom can be considered as ‘charge clouds’ which repel each other
The lowest energy arrangement is when the atoms are as far apart as possible, and this determines the equilibrium molecular shape
Lone pairs repel more than bonding pairs (bond angle gets smaller due to lone pair repulsion)
A multiple bond is treated as through it was a single electron pair
If a lone pair has a choice between an equatorial position and an axial position, it will occupy the equatorial site
This is because in the equatorial position it is repelled less by the two axial bonding pairs than it would be by the three equatorial bonding pairs if it was in the axial position.
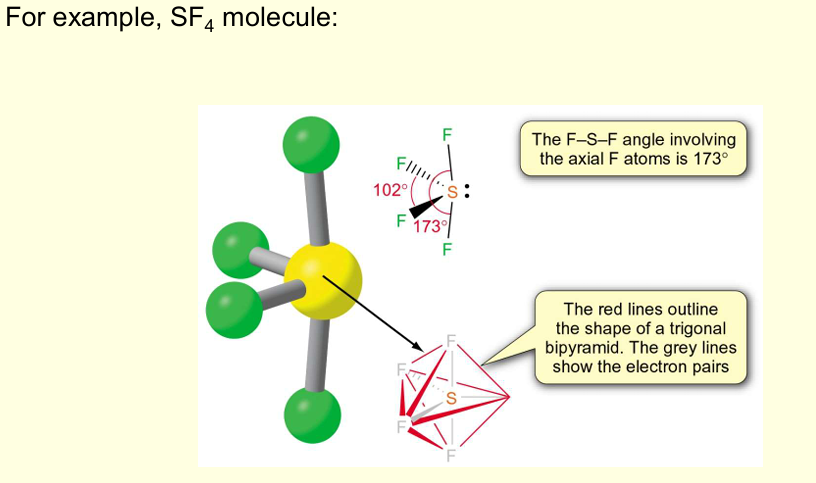
Give the shapes of molecules, bond angles, and examples for 2, 3, 4, and so on.. (till 7) electron pairs around the central atoms with no lone pairs
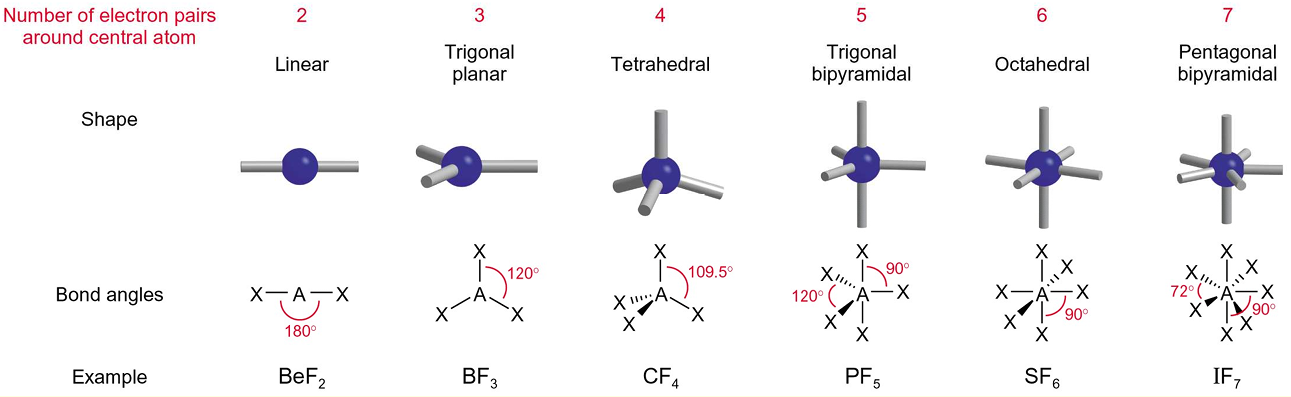
Give the shapes of molecules taking into account of 0, 1, and 2 lone pairs for tetrahedral, trigonal bipyramidal, and octahedral
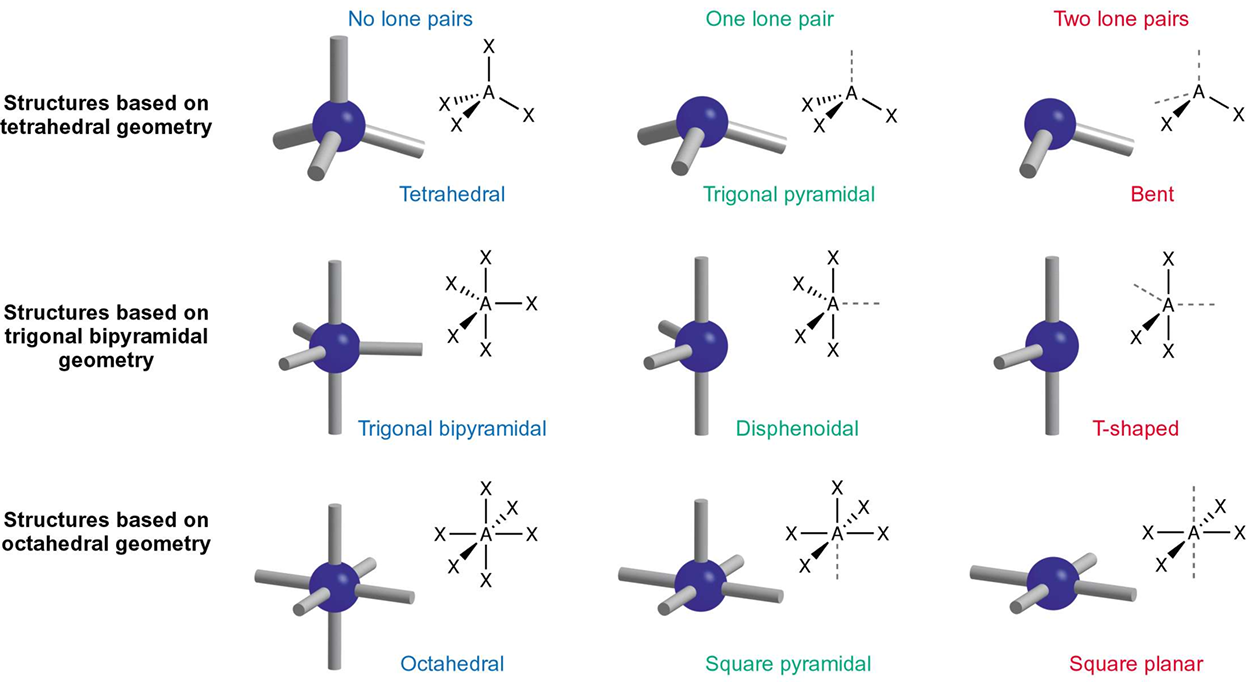
Use the tetrahedral shape to explain why and how bond angle gets smaller after every lone pair
Give examples of molecules with multiple bonds, giving its shape and bond angle
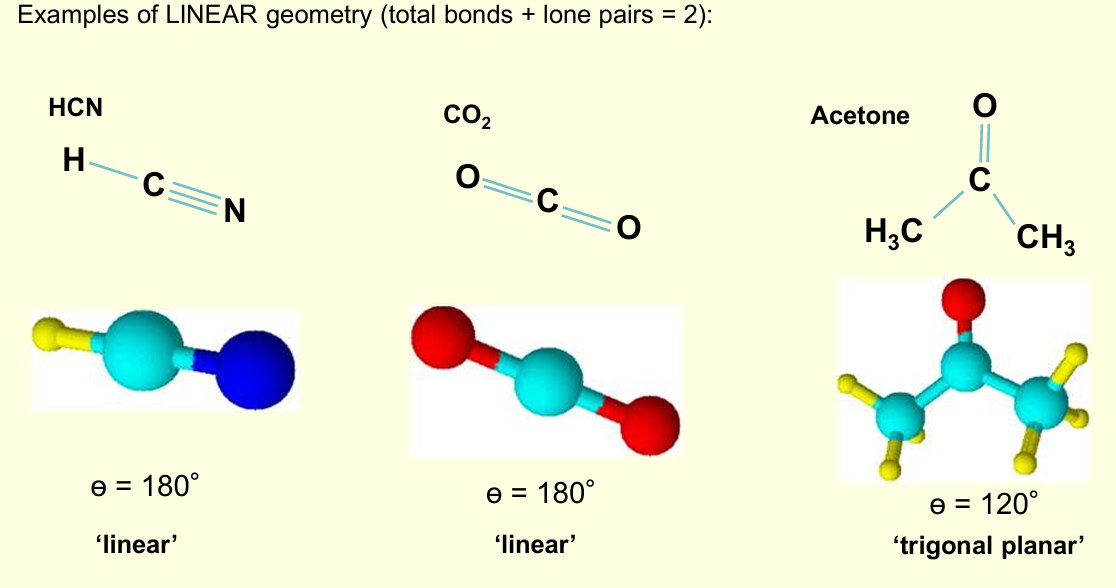
Why does VSEPR ignore resonance?
Because resonance does not change the number or spatial arrangement of electron domains (the number of bonding regions around the central atom stays the same in every resonance form), and multiple bonds act as a single region of electron density
What is the biochemical importance of geometry and bonding, in relation to the VSEPR theory?
How molecules like nucleobases (e.g. guanine) are planar - important in biological function and how they stack in nucleic acids like DNA
Active sites of enzymes specific for substrate of particular size and shape => greater selectivity
Cell receptors/signalling pathways/responses to hormones depend on molecular recognition
Antibody-antigen interactions
If the wrong molecule binds to an enzyme active site this can lead to problems such as inhibition
e.g. CN-, N3-, CO all inhibitors of cytochrome c oxidise active site, as compete with O2 for binding
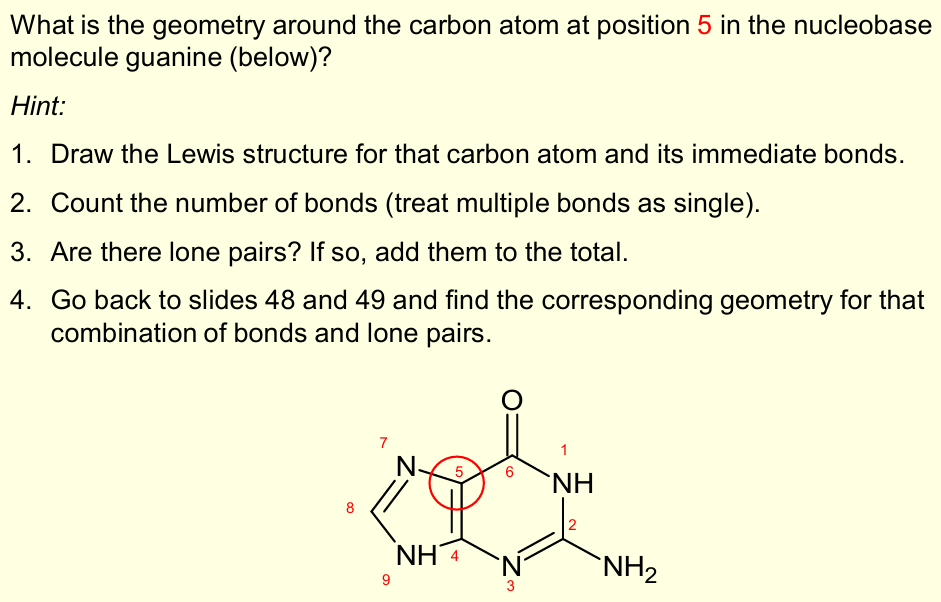
Trigonal Planar
Guanine is planar –important in biological function and how nucleotides stack in DNA.
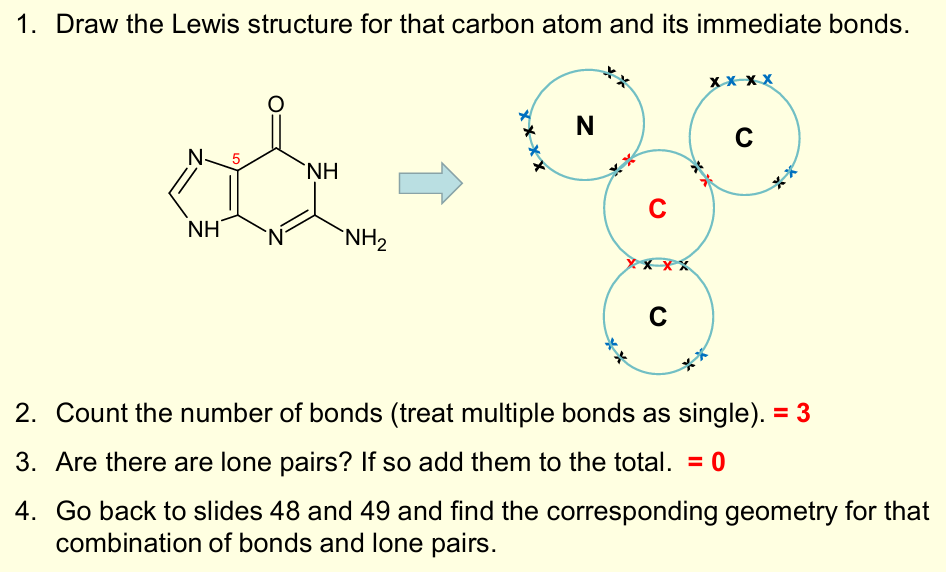
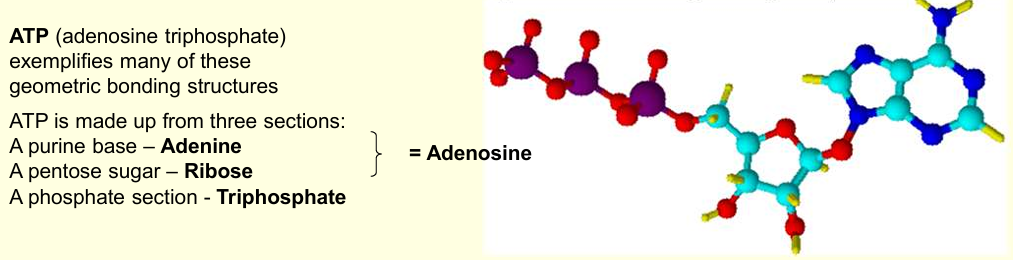
What is the molecules that exemplifies many of these geometric bonding structures?
What are the limitations of the Lewis model of bonding?
Cannot explain hypervalency very satisfactorily
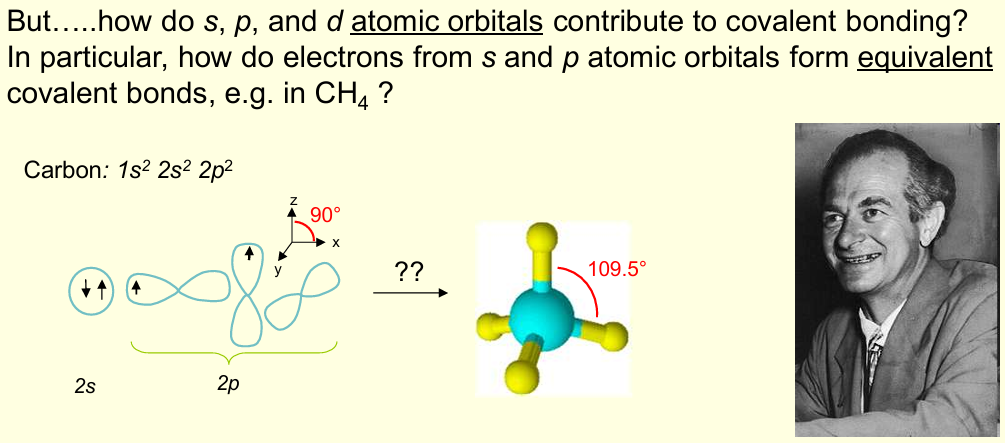
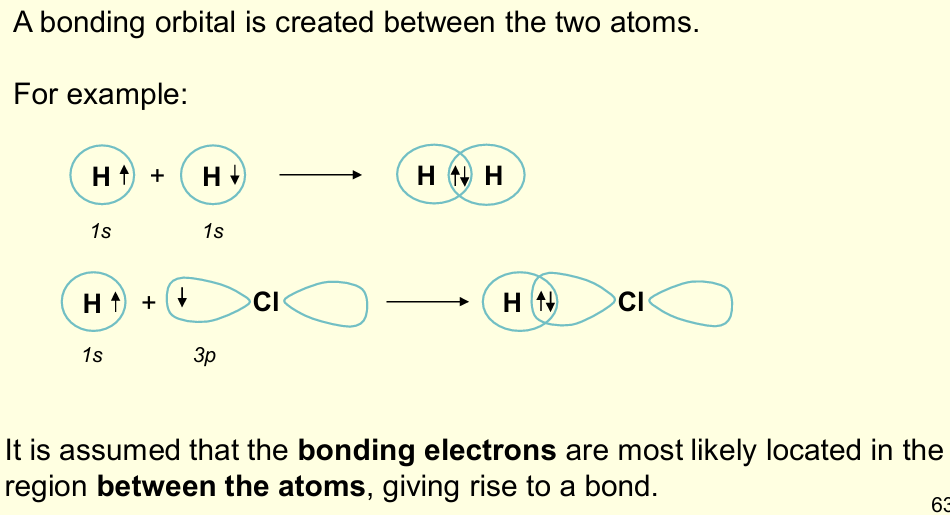
What is the valence bond theory?
Describes how half-filled atomic orbitals on 2 atoms overlap to create a bond containing paired electrons
Linus Pauling proposed an answer to which problem, and what was the answer?
Combinations of the different atomic orbitals to give the same number of equivalent hybrid orbitals, i.e. hybridisation