1.4 Quantum Mechanical Model of Atoms
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Quantum Mechanical Model
• Electrons do not travel in different orbits but are localized in orbitals
• Orbitals are regions of space around the nucleus defined by the probability of finding an electron in that region of space
Heisenberg Uncertainty Principle
• Impossible to know both an electron’s position and its momentum exactly at the same time
Principal Quantum Number
• N
• Describes the average energy of a shell
Azimuthal Quantum Number
• L
• Describes the subshells within a given principle energy level (s, p, d, f)
Magnetic Quantum Number
• Mi
• Specifies the particular orbital within a subshell where an electron is likely to be found at a given moment in time
Spin Quantum Number
• Ms
• Indicates the spin orientation (+1/2 or -1/2) of an electron in an orbital
Electron Configuration
• Electrons fill the principal energy levels and subshells according to increasing energy, determined by n+L rule
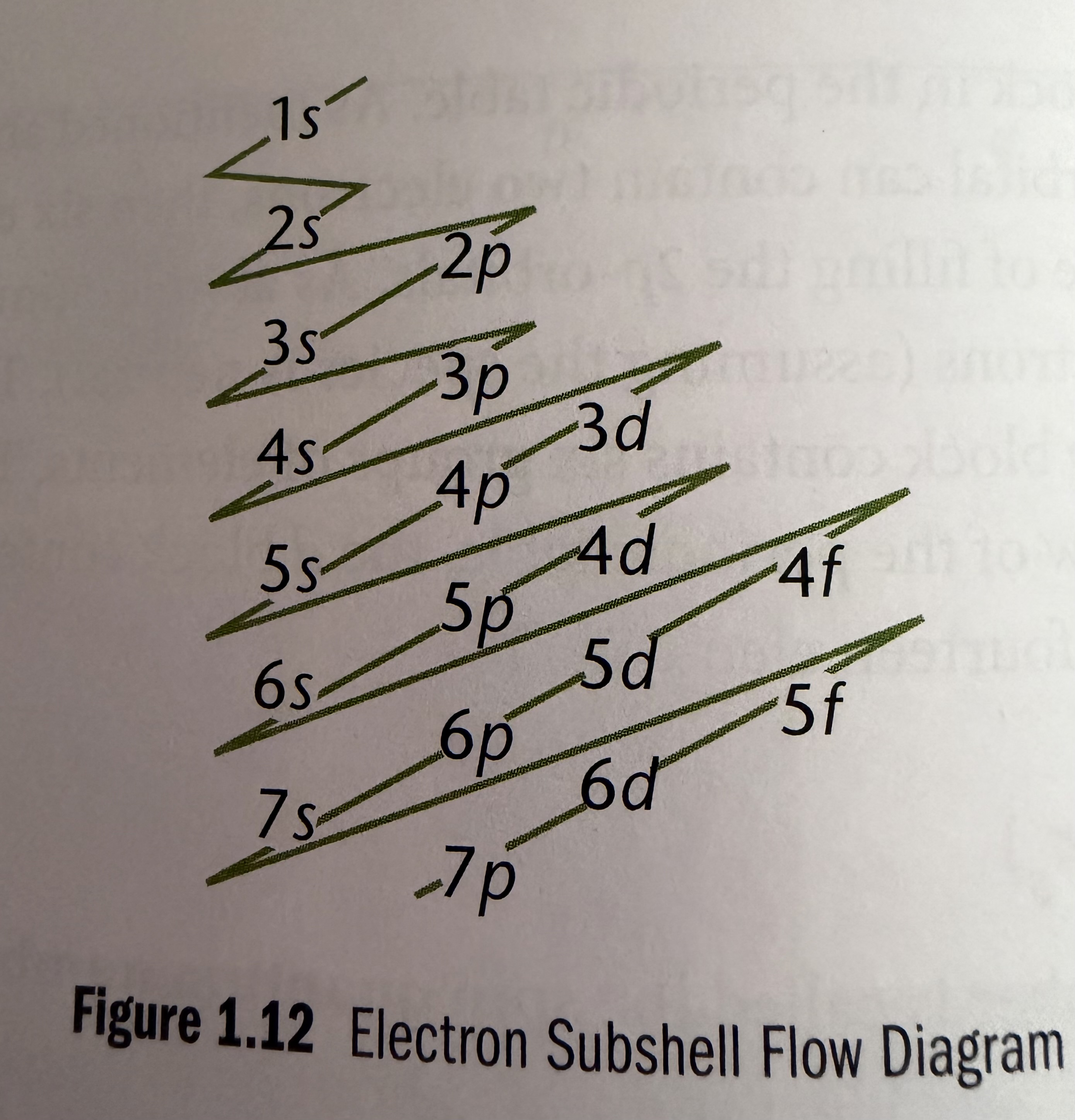
Hund’s Rule
• Subshells with multiple orbitals (p, d, f) fill electrons so that every orbital in a subshell will get one electron before any of them get a second
Paramagnetic
• Unpaired electrons that align with magnetic fields, attracting material to a magnet
Diamagnetic
• Paired electrons that cannot be easily realigned and repelled by magnets
Valence Electrons
• Those electrons in the outermost shell available for interaction (bonding) with other atoms
• For the representative elements (Groups 1, 2, 13-18) valence electrons are in s and/or p orbitals
• For transition elements, valence electrons are found in s, d, or f orbitals