Chapter 15: Amines
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
Amine
classified as 1, 2, 3 depending on the number of carbon groups bonded to nitrogen
Aliphatic amine
all carbons bonded to nitrogen are derived from alkyl groups
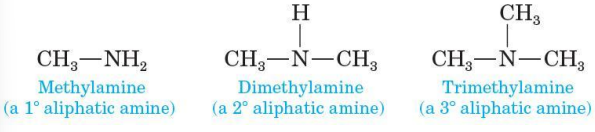
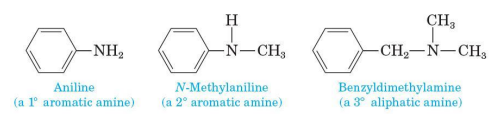
Aromatic amine
one or more of the groups bonded to nitrogen are aryl groups
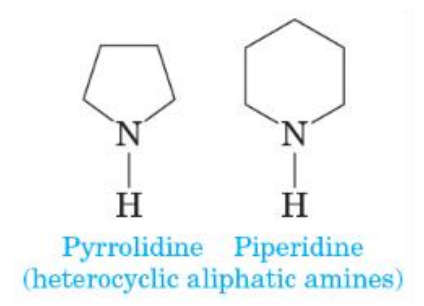
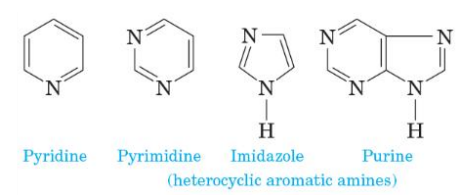
Heterocyclic amine
an amine in which the nitrogen atom is part of a ring
Heterocyclic aliphatic amine
a heterocyclic amine in which the ring is saturated (has no c=c bonds)
Heterocyclic aromatic amine
amine nitrogen is part of an aromatic ring
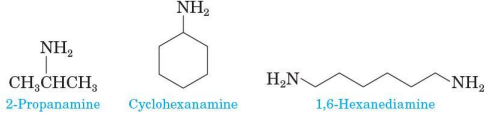
Aliphatic amine IUPAC naming
drop the final -e of the parent alkane and replace it by -amine
use a number to locate the amino group on the parent chain
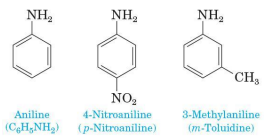
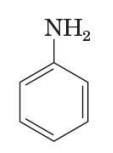
Aniline (aromatic amine) IUPAC naming
retain the common name aniline for C6H5NH2 (the simplest aromatic aniline
name simple derivatives of aniline by using numbers to locate substituents of use the prefixes ortho, meta, para
C6H5NH2
simplest aromatic amine
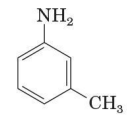
Toluidine
one of the several derivatives of aniline widely uses common names
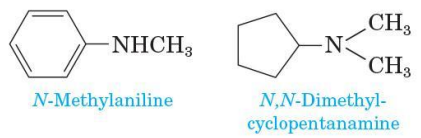
Unsymmetrical secondary and tertiary amine IUPAC naming
name unsymmetrical secondary and tertiary amines as N-substituted primary amines
take the largest group bonded to nitrogen as the parent amine
name the smaller groups bonded to nitrogen and show their location by using the prefix N-(indicating that they are bonded to nitrogen)
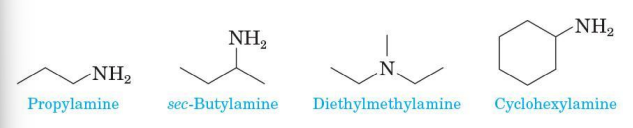
Common names
for most aliphatic amines, list the groups bonded to nitrogen in alphabetical order in one word ending in the suffix -amine
Amine salt
when 4 atoms or groups of atoms are bonded to a nitrogen atom, as for examples CH3NH3+ nitrogen bears a positive charge and is associated with anion as a salt
nomenclature: replace the ending -amine (or aniline or pyridine) by -ammonium (or anilinium or pyridinium) and add the name of the anion
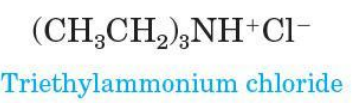
Low-molecular-weight amines
like ammonia, have vey sharp penetrating odors
ex: trimethylamine - pungent principle in the smell of rotting fish
other pungent amines: 1,4-butanediamine (putrescine) and 1,5-pentanediamine (cadaverine)
Trimethylamine
pungent principle in the smell of rotting fish
1,4-butanediamine (putrescine)
rotting fish, decaying meat smell
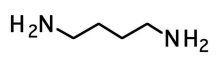
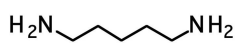
1,5-pentanamine (cadaverine)
decaying corpses smell
Amines
are polar compounds
1° and 2° amines
have N-H bonds
can form hydrogen bonds with one another
3° amines
have no N-H bond
cannot form hydrogen bonds with one another
N-H- - -H hydrogen bond
weaker than O-H- - -O hydrogen bond because the difference in electronegativity between N and H (3.0 -2.1=0.9) is less than that between O and H (3.5-2.1=1.4)
All classes of amines
form hydrogen bonds with water
more soluble in water than are hydrocarbons of comparable molecular weight
Most lo-molecular-weight amines
completely soluble in water
Higher-molecular-weight amines
only moderately soluble in water or insoluble
Amines
like ammonia, they are weak bases and its aqueous solutions are basic
Acid-base reaction between an amine and water
involves transfer of a proton from water to the amine
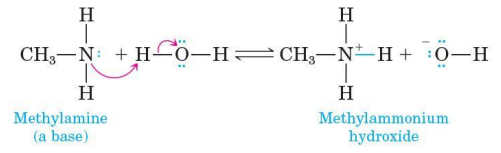
Aliphatic amines
have about the same base strength and are slightly stronger bases than NH3
weak bases by comparison with inorganic bases such as NaOH, they are strong bases among organic compounds
Aromatic and heterocyclic aromatic amines
considerable weaker bases than aliphatic amines
Ammonia (NH3)
weaker base against aliphatic amines but stronger than aromatic
Basicity of amines
with it, we can determine which form of an amine exists in body fluids
7.40
in a normal, healthy person, the pH of blood is approximately _____ which is slightly basic
Aliphatic amine
if dissolved in blood, it is present predominantly as its protonated (conjugated acid) form
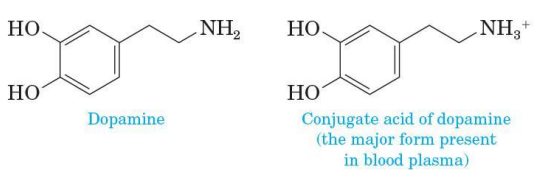
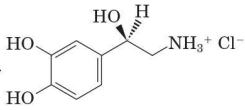
Conjugate acid of dopamine
major form present in blood plasma
Basicity
the most important chemical property of amines
Amines
whether soluble or insoluble in water, react quantitatively with string acids to form water-soluble salts
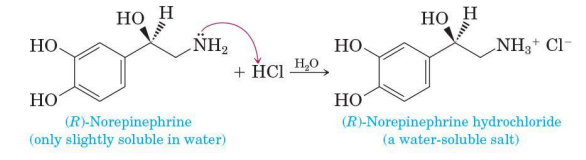
(R)-norepinephrine
only slightly soluble in water
(R)-norepinephrine hydrochloride
a water soluble salt