Biochem HW 2 (Chapter 4)
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Which of the following statements are true concerning peptide bonds?
All of the above.
Match each amino acid with the appropriate side-chain type.
Ile, Asp, Arg, Tyr, Met, Trp
Acidic, Basic, Branched side chain, Hydroxyl- containing, Sulfur containing, Non-polar aromatic
Ile: branched side chain
Asp: acidic
Arg: basic
Tyr: hydroxyl-containing
Met: sulfur containing
Trp: non-polar aromatic
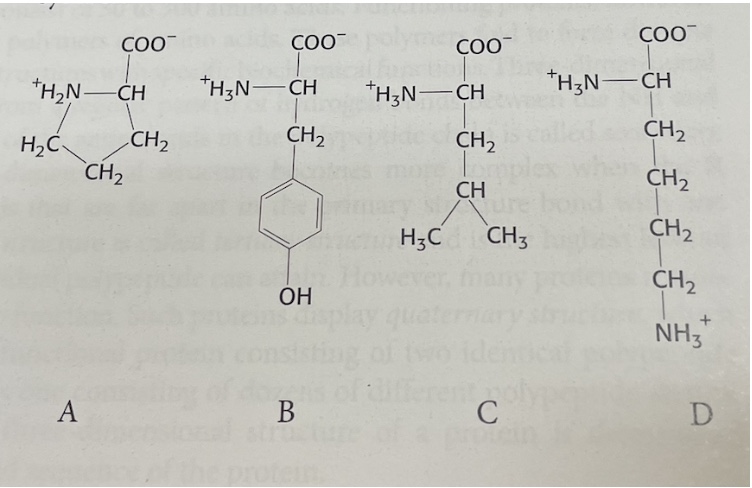
Examine the following four amino acids. Match them with their correct one-letter abbreviation.
A,B,C,D
P,K,Y,L
A: P
B: Y
C: L
D: K
Which of the following forces are involved in maintaining the quaternary structure of a protein?
hydrophobic interactions, hydrogen bonds, disulfide bonds, and
ionic interactions
In each of the following pairs of amino acids, identify which amino acid would be most soluble in water:
Glu or Leu:
Cys or Phe:
Tyr or Ala:
Trp or His:
Glu or Leu: Glu
Cys or Phe: Cys
Tyr or Ala: Tyr
Trp or His: His
Which amino acids have positively charged R groups at pH 7?
Both Arg and Lys
The beta-pleated sheet secondary structure of proteins is most stabilized by:
hydrogen bonding between peptide backbone groups.
Examine the following peptide:
Thr-Glu-Pro-Ile-Val-Ala-Pro-Met-Glu-Tyr-Gly-Lys
Write the sequence using one letter abbreviations (enter as all capital letters):
Estimate the net charge at pH 7 (enter as a whole number and include either a + or - sign prior to the number; i.e. -3 or +3):
Estimate the net charge at pH 12 (enter as a whole number):
TEPIVAPMEYGK, -1, -4
Match the terms with the appropriate description.
Terms:
Primary structure
Peptide bond
Disulfide bond
Secondary structure
Definitions:
Sequence of amino acids in a protein.
The bond responsible for primary structure.
Forms between two Cys amino acids.
Formed by hydrogen bonds between the backbone of amino acids.
Primary structure: Sequence of amino acids in a protein.
Peptide bond: The bond responsible for primary structure.
Disulfide bond: Forms between two Cys amino acids.
Secondary structure: Formed by hydrogen bonds between the backbone of amino acids.
Hydrophilic amino acids tend to reside _____________ of proteins; whereas hydrophobic amino acids tend to reside ______________ of proteins.
on the surface; in the core
Most amino acids are found as the L-isomer in nature.
True
At physiological pH (7.4), the carboxylic acid group of an amino acid will be ________, while the amino group will be ________, yielding the zwitterion form.
deprotonated; protonated
The side chain of ________ has a pKa in the physiological pH range and is therefore often involved in proton transfer during enzymatic catalysis.
Histidine
At pH=0, the net charge on an amino acid will be negative. (Hint: Draw an amino acid and look at the charges on it to help you answer this question.)
False
For the hexapeptide below, which amino acids would most likely be on the surface of a protein?
Ile-Tyr-Leu-Val-Asp-Glu
All of the above.
Which of the following amino acids would carry a net negative charge at pH 7.0?
Both
Which of the following could be expected to form an ion pair in protein tertiary structure?
Asp + Arg
What is the net charge on the peptide below at pH 7? (Always be sure to consider the N-terminal and C-terminal of the peptide)
Gly-Lys-Tyr-His-Val
+1
A protein with quaternary structure…
contains more than one subunit.
In a neutral solution, most amino acids exist as:
zwitterions.
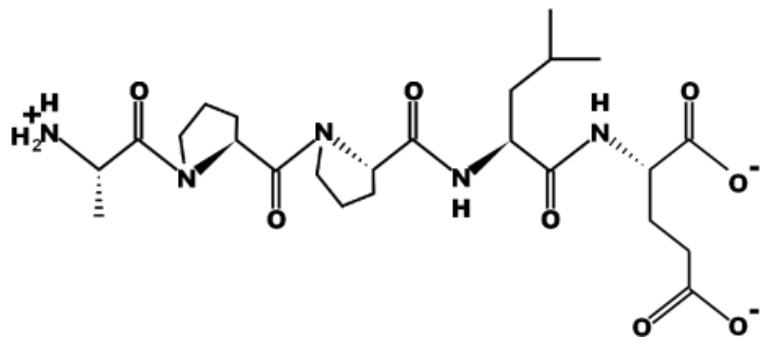
Identify the N-terminal and C-terminal amino acids in the peptide below:
N-terminal: Ala and C-terminal: Glu
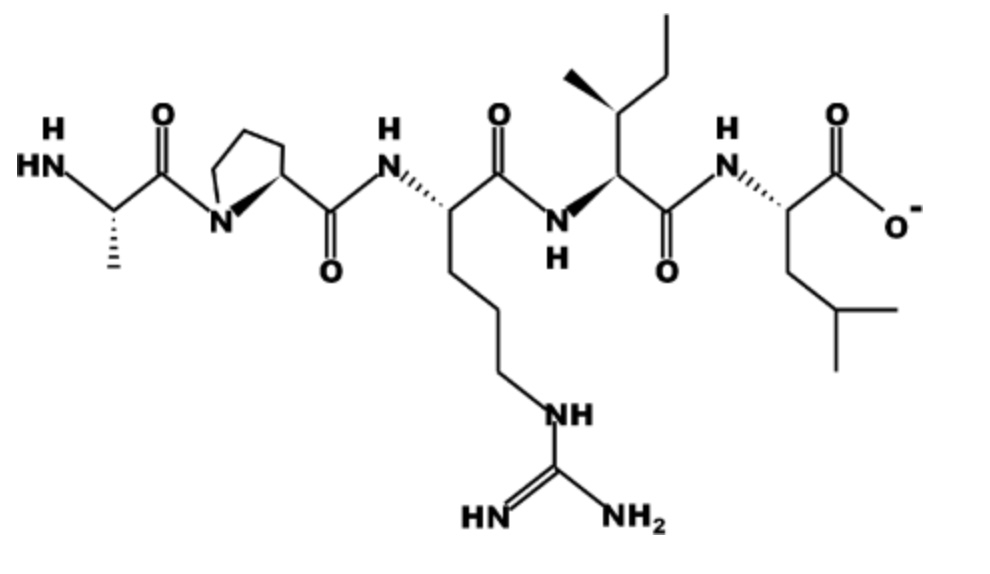
Part A: How many amino acids are in the peptide above? (Enter your answer as a whole number.)
Part B: Identify the N-terminal amino acid in the peptide? (Enter your answer as the 3 letter abbreviation of the amino acid).
Part C; Identify the C-terminal amino acid in the peptide? (Enter your answer as the 3 letter abbreviation of the amino acid).
Part D: Input the entire sequence of the peptide using only the one letter codes. (Please enter as uppercase letters and do not include punctuation.)
Part E: Is the peptide drawn at an acidic (below pH 7) or basic (above pH 7) pH? Hint: Look at what groups are protonated/deprotonated. (Enter only 'acidic' or 'basic' as your answer).
Part A: 5 Part B: Ala Part C: Leu Part D: APRIL Part E: basic
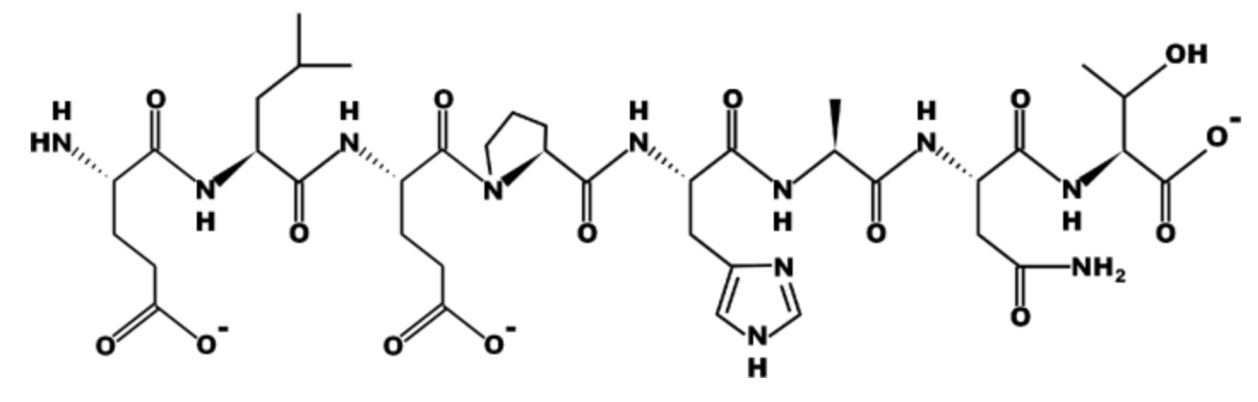
Part A: Input the sequence of the above peptide using only the one letter codes. DO NOT INCLUDE PUNCTUATION. DO NOT MIX LETTER CASE.
Part B: Is the peptide drawn at an acidic (below pH 7) or basic (above pH 7) pH? (Enter only 'acidic' or 'basic' as your answer).
Part A: ELEPHANT
Part B: Basic