chemistry - extracting metals & equilibria: reversible reactions & equilibria (4.13 - 4.17)
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
4.13 are chemical reactions reversible/irreversible?
reversible (sometimes?)
4.13 reversible reaction symbol
⇌
4.13 how can direction (forward/backward) of some reversible reactions be altered?
by changing reaction conditions
4.14 dynamic equilibrium
forward & backward reactions still occurring (dynamic)
% of reactants & products no longer changing - substances in balance (equilibrium)
4.15 formation of ammonia
reversible reaction between nitrogen (from air) & hydrogen (from natural gas)
can reach dynamic equilibrium
4.16 conditions for Haber process
temp. 450°C
pressure 200 atmospheres
iron catalyst
what does equilibrium position shift to do?
shifts to oppose changes to system
4.17 change in temperature effect on position of dynamic equilibrium
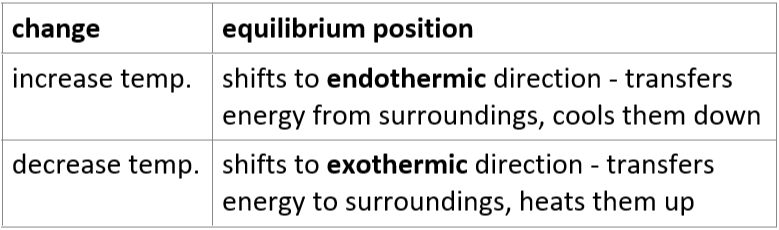
4.17 change in pressure effect on position of dynamic equilibrium

4.17 change in concentration effect on position of dynamic equilibrium
