Nature of covalent and dative covalent bonds (3.1.3.2) + macromolecular structures
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
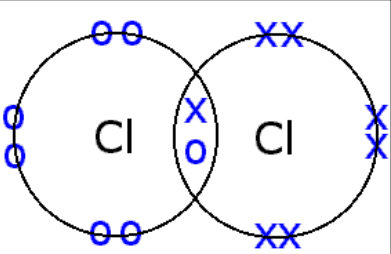
What is a covalent bond?
Covalent bonds occur between two non-metals.
Electrostatic attraction between the nuclei of two atoms and the bonding electrons of their outer shells.
How can you represent covalent bonds?
Dot and cross diagram
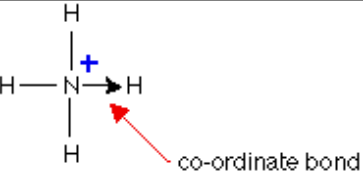
What are dative/coordinate bonds and how are they indicated?
Dative bonds - when the electrons in a shared pair are supplied from a single atom.
It is indicated using an arrow from the lone electron pair
What are the physical properties of simple molecular compounds?
Melting and boiling points
They have low MP and BP as they have intermolecular forces that require low amounts of energy to break
Conductivity
Very poor conductors as their structure contains no charged particles
What are the physical properties of a macromolecular compounds?
Melting and boiling points
They have many strong covalent bonds that require a lot of energy to break so they have high MP and BP
Conductivity
Most don’t conduct electricity except for graphite