PHOrgChem (Lecture) | Module 6 (Part 3: ORGANIC REACTIONS ONLY)
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
91 Terms
Organic Reactions
They are chemical reactions involving organic compounds which primarily consist of Carbon and Hydrogen, often with Oxygen, Nitrogen, Sulfur, and other elements.
Organic Reactions
These reactions plays a crucial role in biochemistry, pharmaceutical, material sciences and industrial chemistry
Organic Reactions
These are essential in creating drugs, plastics dyes, and many other materials.
Organic Reactions
Understanding their mechanism helps in designing efficient synthetic pathways.
Nature of reactants, Catalyst, Temperature and Pressure, and Solvent Used
FACTORS THAT INFLUENCES ORGANIC REACTIONS
Nature of reactants
FACTORS THAT INFLUENCES ORGANIC REACTIONS: affects stability and reactivity of molecules
Catalyst
FACTORS THAT INFLUENCES ORGANIC REACTIONS: Used to speed up reactions without being consumed
Temperature and Pressure
FACTORS THAT INFLUENCES ORGANIC REACTIONS: Affects reaction rate and equilibrium
Solvent Used
FACTORS THAT INFLUENCES ORGANIC REACTIONS: Stabilizes intermediate or influence reaction pathways
Substitution Reactions, Addition and Elimination Reactions, Oxidation-Reduction (Redox) Reactions, and Rearrangement Reactions
TYPES OF ORGANIC REACTIONS
Substitution Reactions
One functional group in a molecule is replaced by another.
Substitution Reactions
Example: Nucleophilic Substitution (SN1 & SN2) – Halogenoalkanes react with nucleophiles.
Substitution Reactions
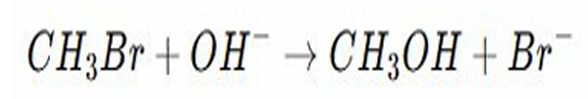
Nucleophile
SUBSTITUTION REACTIONS:
EXAMPLE REACTION
In this reaction bromo methane under undergoes nucleophilic substitution with hydroxide ion
The hydroxide ion is a strong ________ and attacks the carbon bonded to bromine, since bromine is more electronegative it takes the bonding electrons and leave as a bromide ion
The hydroxide ion replace bromine forming methanol
Electronegative
SUBSTITUTION REACTIONS:
EXAMPLE REACTION
In this reaction bromo methane under undergoes nucleophilic substitution with hydroxide ion
The hydroxide ion is a strong nucleophile and attacks the carbon bonded to bromine, since bromine is more ______________ it takes the bonding electrons and leave as a bromide ion
The hydroxide ion replace bromine forming methanol
SN1 (Substitution Nucleophilic Unimolecular) Reaction, and SN2 (Substitution Nucleophilic Bimolecular) Reaction
TWO TYPES OF SUBSTITUTION REACTION
Substitution Nucleophilic Unimolecular
SN1 stands for?
Substitution Nucleophilic Bimolecular
SN2 stands for?
SN1 (Substitution Nucleophilic Unimolecular) Reaction
A two-step reaction where the rate determining step (slow step) involves the unimolecular departure of the leaving group, forming a carbocation intermediate.
SN1 (Substitution Nucleophilic Unimolecular) Reaction
One step at a time
SN1 (Substitution Nucleophilic Unimolecular) Reaction
First, the leaving group like Chlorine, Bromine, or Iodine leaves, forming a carbon cation then the nucleophile attacks.
SN1 (Substitution Nucleophilic Unimolecular) Reaction
The rate depends on one molecule.
SN1 (Substitution Nucleophilic Unimolecular) Reaction
The reaction speed depends only on the concentration of the substrate not the nucleophile.
SN1 (Substitution Nucleophilic Unimolecular) Reaction
Prefers stable cations works best with tertiary carbons because they stabilize the carbon cation
Tertiary Carbons
SN1 prefers stable cations works best with ___________ because they stabilize the carbon cation
SN1 (Substitution Nucleophilic Unimolecular) Reaction
Forms a racemic mixture, since the nucleophile can attack from either side, so a mix of products forms
Racemic Mixture
SN1 forms a ______________, since the nucleophile can attack from either side, so a mix of products forms
SN1 (Substitution Nucleophilic Unimolecular) Reaction
Common in polar protic solvents, like solvents with hydrogen bonding such as water and alcohol
SN1 (Substitution Nucleophilic Unimolecular) Reaction
If it follows this mechanism, the Carbon Bromine bonds breaks first forming a carbon cation then the hydroxyl attaches in the second steps
SN2 (Substitution Nucleophilic Bimolecular) Reaction
A one-step reaction where the nucleophile attacks the substrate at the same time as the leaving group departs, resulting in a concerted mechanism
SN2 (Substitution Nucleophilic Bimolecular) Reaction
A one step reaction, where the nucleophile directly attacks the carbon, forcing the leaving group out at the same time
SN2 (Substitution Nucleophilic Bimolecular) Reaction
Rate depends on the two molecules
SN2 (Substitution Nucleophilic Bimolecular) Reaction
Both the substrate and the nucleophile affects the reaction speed
SN2 (Substitution Nucleophilic Bimolecular) Reaction
Prefer less hindered carbons works best with primary or secondary carbon because the static hindrance slows it down, causes inversion or a backside attack
SN2 (Substitution Nucleophilic Bimolecular) Reaction
The product has opposite configuration
SN2 (Substitution Nucleophilic Bimolecular) Reaction
Common in aprotic solvents, works well in solvents like acetone or DMSO which don't form hydrogen bonds
SN2 (Substitution Nucleophilic Bimolecular) Reaction
If it follows this mechanism, the hydroxide attacks from the opposite side of the leaving bromine leading to a one step reaction with inversion of configuration
SN1 (Substitution Nucleophilic Unimolecular) Reaction
It is equals to stepwise and stable carbocation
SN2 (Substitution Nucleophilic Bimolecular) Reaction
It is single step and steric hindrance matters
Addition Reactions
Atoms or groups are added to a molecule, typically occurring in compounds with double or triple bonds.
Addition Reactions
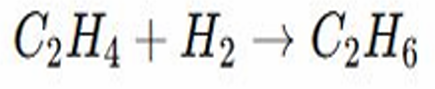
Addition Reactions
Example: Hydrogenation of Alkenes – Ethene reacts with hydrogen to form ethane. ○
Ethene has a carbon carbon double bond meaning there are two pairs of electrons between the carbon atoms
Hydrogenation of Alkenes
In the presence of a catalyst like platinum, palladium, or nickel, hydrogen gas is added to the molecule, so the double bond breaks and each carbon gains a hydrogen atom, converting ethene into ethane which has only single bonds
Hydrogenation
This is called ___________ - commonly used in the food industry, ex. Converting vegetable oils into margarine
Elimination Reactions
The removal of atoms or groups from a molecule, often forming a double bond.
Elimination Reactions
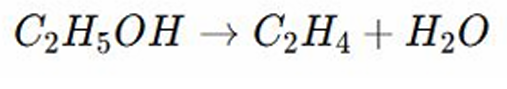
Elimination Reactions
Example: Dehydration of Alcohols – Ethanol loses water to form ethene.
Ethanol undergoes dehydration or loss of water in the presence of an acid catalyst . Example: concentrated sulfuric acid
A hydrogen atom and the OH group is removed from adjacent carbon atoms
This forms ethene which has a carbon-carbon double bond and water as a by product
This is an elimination reaction because atoms are removed from the molecule rather than added
Addition Reactions
Example: Hydrogenation of Alkenes
Elimination Reactions
Example: Dehydration of Alcohols
Oxidation
It involves the loss of electrons (or gain of oxygen)
Reduction
It involves the gain of electrons (or loss of oxygen).
Oxidation-Reduction (Redox) Reactions
Example: Oxidation of Alcohols – Ethanol oxidizes to ethanoic acid.
Ethanol is oxidized using an oxidizing agent such as potassium dichromate and acid
It first forms ethanal, as an intermediate, further oxidation leads to ethanoic acid, commonly known as acetic acid or vinegar
The oxygen atoms supplied by the oxidizing agent
Alcohols oxidize to aldehyde and then to carboxylic acids
Potassium dichromate, and Acid
Oxidizing Agent
Ethanal
Example: Oxidation of Alcohols – Ethanol oxidizes to ethanoic acid.
Ethanol is oxidized using an oxidizing agent such as potassium dichromate and acid
It first forms ______, as an intermediate, further oxidation leads to ethanoic acid, commonly known as acetic acid or vinegar
Ethanoic Acid
Example: Oxidation of Alcohols – Ethanol oxidizes to ethanoic acid.
Ethanol is oxidized using an oxidizing agent such as potassium dichromate and acid
It first forms ethanal, as an intermediate, further oxidation leads to __________, commonly known as acetic acid or vinegar
Oxidation-Reduction (Redox) Reactions
Example: Oxidation of Alcohols
Rearrangement Reactions
The structure of a molecule is rearranged to form an isomer.
Rearrangement Reactions
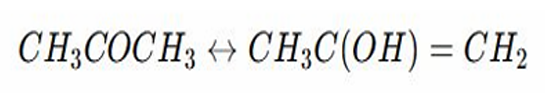
Rearrangement Reactions
Example: Keto Enol Tautomerism – Interconversion of ketones and enols.
Propanone exist in equilibrium with its enol form
This is tautomeric shift where a hydrogen atom moves and a double bond is rearranged
The keto form is usually more stable than the enol form, however the enol form can be important in reactions like electrophilic addition
This type of rearrangement is crucial in biological and organic chemistry affecting stability and reactivity
Rearrangement Reactions
Example: Keto Enol Tautomerism
Substitution Reaction (Halogenation), and Combustion Reaction
REACTION OF ALKANES
Substitution Reaction (Halogenation)
Example: CH₄ + Cl₂ CH₃Cl + HCl (UV light)
The carbon-hydrogen bond in methane is broken, a chlorine molecule is split by the by the UV light into two chlorine radicals
One chlorine replaces a hydrogen atom in CH4 forming methyl chloride and hydrochloride
If excess chlorine is present, further substitution can occur forming methylene chloride, chloroform, and carbon tetrachloride
Methylene chloride, Chloroform, and Carbon tetrachloride
SUBSTITUTION REACTION (HALOGENATION) IN ALKANES
Example: CH₄ + Cl₂ CH₃Cl + HCl (UV light)
The carbon-hydrogen bond in methane is broken, a chlorine molecule is split by the by the UV light into two chlorine radicals
One chlorine replaces a hydrogen atom in CH4 forming methyl chloride and hydrochloride
If excess chlorine is present, further substitution can occur forming __________, __________, and ___________
Combustion Reaction
Example: CH₄ + 2O₂ CO₂ + 2H₂O
Methane reacts with oxygen in a highly exothermic reaction
Complete combustion produces carbon dioxide and water
If oxygen is limited, carbon monoxide or carbon may form instead
Carbon dioxide, and Water
COMBUSTION REACTION IN ALKANES
Example: CH₄ + 2O₂ CO₂ + 2H₂O
Methane reacts with oxygen in a highly exothermic reaction
Complete combustion produces __________, and ________
If oxygen is limited, carbon monoxide or carbon may form instead
Carbon monoxide, or Carbon
Example: CH₄ + 2O₂ CO₂ + 2H₂O
Methane reacts with oxygen in a highly exothermic reaction
Complete combustion produces carbon dioxide and water
If oxygen is limited, ________, or _______ may form instead
Hydrogenation, Halogenation, Hydrohalogenation, and Hydration
REACTION OF ALKENES
Hydrogenation
C₂H₄ + H₂ C₂H₆ (Ni catalyst)
The double bond in ethene is broken, two hydrogen atoms are added across the bond converting ethene into ethane, a saturated hydrocarbon
Halogenation
C₂H₄ + Br₂ C₂H₄Br₂
The pi bond in the double bond is broken, two bromine atoms are added to carbon atoms forming 1,2 dibromoethane
The reddish brown bromine solution becomes colorless which is a test for unsaturation
Hydrohalogenation
C₂H₄ + HCl C₂H₅Cl
The double bond breaks and the hydrogen from hydrochloride, bonds with one carbon and chlorine attaches to another carbon forming chloroethane
In Markovnikov's rule applies, the hydrogen attaches to the carbon that already has more hydrogens
Markovnikov's Rule
What rule applies on Hydrohalogenation (an organic reaction on ALKENES) the hydrogen attaches to the carbon that already has more hydrogens
Hydration
C₂H₄ + H₂O C₂H₅OH (H₂SO₄ catalyst)
The double bond is broken and hydrogen attaches to one carbon while hydroxide is attaches to other
Ethanol is formed, which is a key industrial alcohol
Ethanol
This is formed in Hydration (an organic reaction in ALKENES) which is a key industrial alcohol
Addition of Hydrogen, and Oxidation (Combustion)
REACTIONS OF ALKYNES
Addition of Hydrogen
C₂H₂ + 2H₂ C₂H₆
The hydrogenation of alkynes, the triple bond in ethyne is completely hydrogenated converted to single bonds, two molecules of hydrogen are added, converting ethyne to ethene to ethane
Oxidation (Combustion)
C₂H₂ + O₂ CO₂ + H₂O
Acetylene burns in oxygen releasing the intense heat forming carbon dioxide and water
This is used in oxyacetylene welding torches due to high temperature
Oxyacetylene Welding Torches
Oxidation (Combustion) is used in?
Substitution
Aromatic hydrocarbons undergo ________ instead of addition due to benzene stable structure
Halogenation, Nitration, and Friedel-Crafts Alkylation
REACTIONS OF AROMATIC HYDROCARBONS (BENZENES)
Halogenation
C₆H₆ + Cl₂ C₆H₅Cl + HCl (FeCl₃ catalyst)
One halogen from benzene is replace by a chlorine from chloride
Ferric chloride catalyst helps generates chlorine that attacks the benzene
Nitration
C₆H₆ + HNO₃ C₆H₅NO₂ + H₂O (H₂SO₄ catalyst)
A nitronium ion is generated by nitric acid and and sulfuric acid
Nitrogen dioxide replaces a hydrogen, forming nitrobenzene
Friedel-Crafts Alkylation
C₆H₆ + CH₃Cl C₆H₅CH₃ + HCl (AlCl₃ catalyst)
The methyl cation is generated by the chloromethane and aluminum trichloride
Methyl replaces a hydrogen in benzene forming toluene
Nucleophilic Substitution (SN1 and SN2), and Elimination (E1 and E2)
REACTIONS OF ALKYL HALIDES
Nucleophilic Substitution (SN1 and SN2)
CH₃Br + OH⁻ CH₃OH + Br⁻
The bromine is replace by OH, converting methyl bromide into methanol
SN1 Mechanism
This mechanism is a two step process with a carbocation intermediate
SN2 Mechanism
This mechanism is a one step direct attack by hydroxyl
Elimination (E1 and E2)
CH₃CH₂Br + NaOH (heat) CH₂=CH₂ + HBr
Instead of substitution, hydrogen and bromine are remove from adjacent carbon atoms, which forms an alkene which is ethene, by an elimination of hydrogen halide
Alkanes
They react by a substitution due to strong carbon and hydrogen bonds
Alkenes, and Alkynes
They react by a addition due to reactive double and triple bonds
Benzenes
They undergo electrophilic substitution due to resonance stability
Alkyl Halides
They undergo nucleophilic substitution or elimination depending on conditions