R3.4.6 & R.3.47 Lewis acids and bases
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
Lewis acid
Electron pair acceptor
2
New cards
Lewis base
Electron pair donor
3
New cards
How do Lewis acids and bases react?
Coordinate covalent bond formed between Lewis acid and base
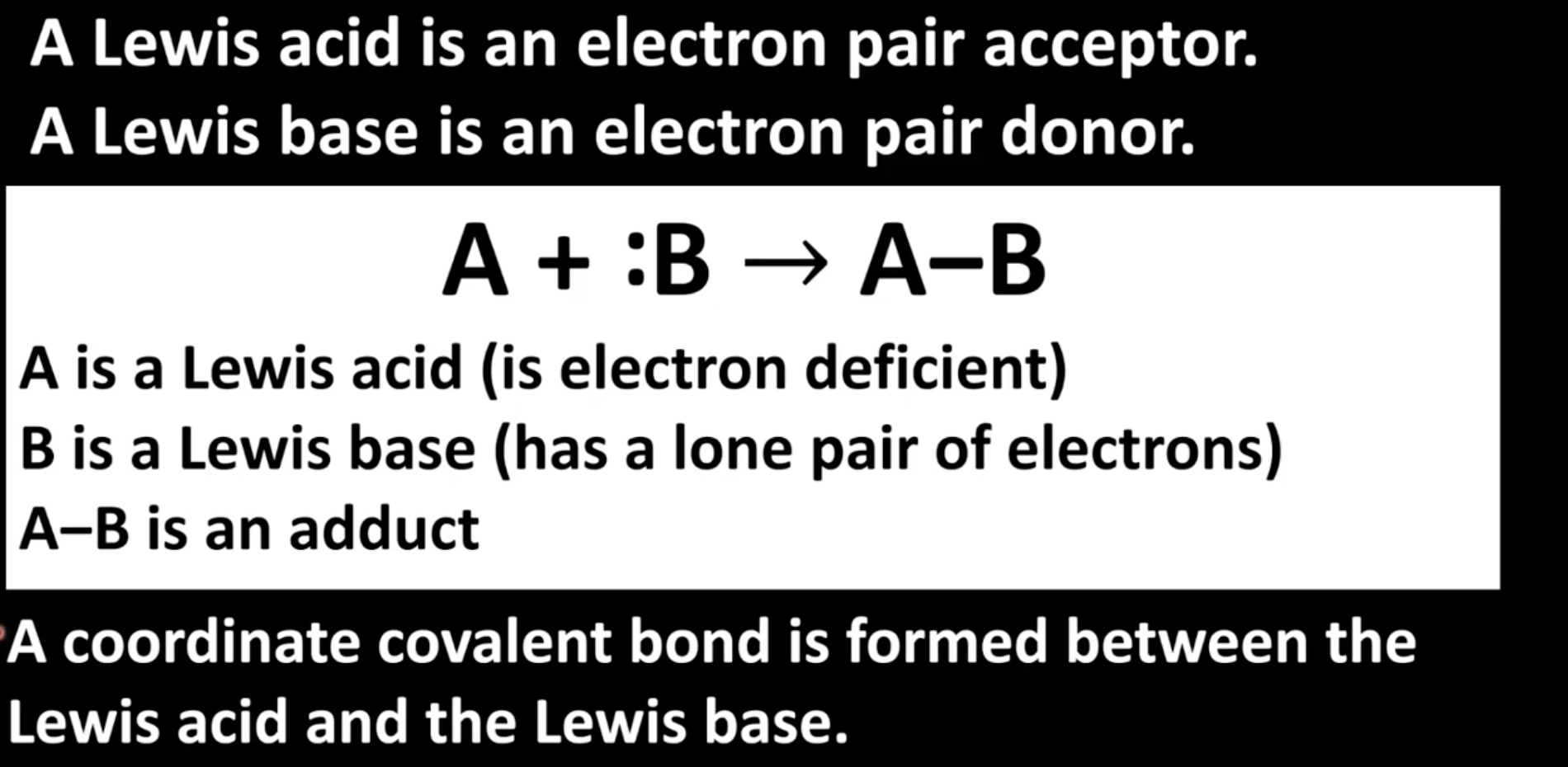
4
New cards
Electrophiles
Electron deficient species that accept lone pairs of electrons
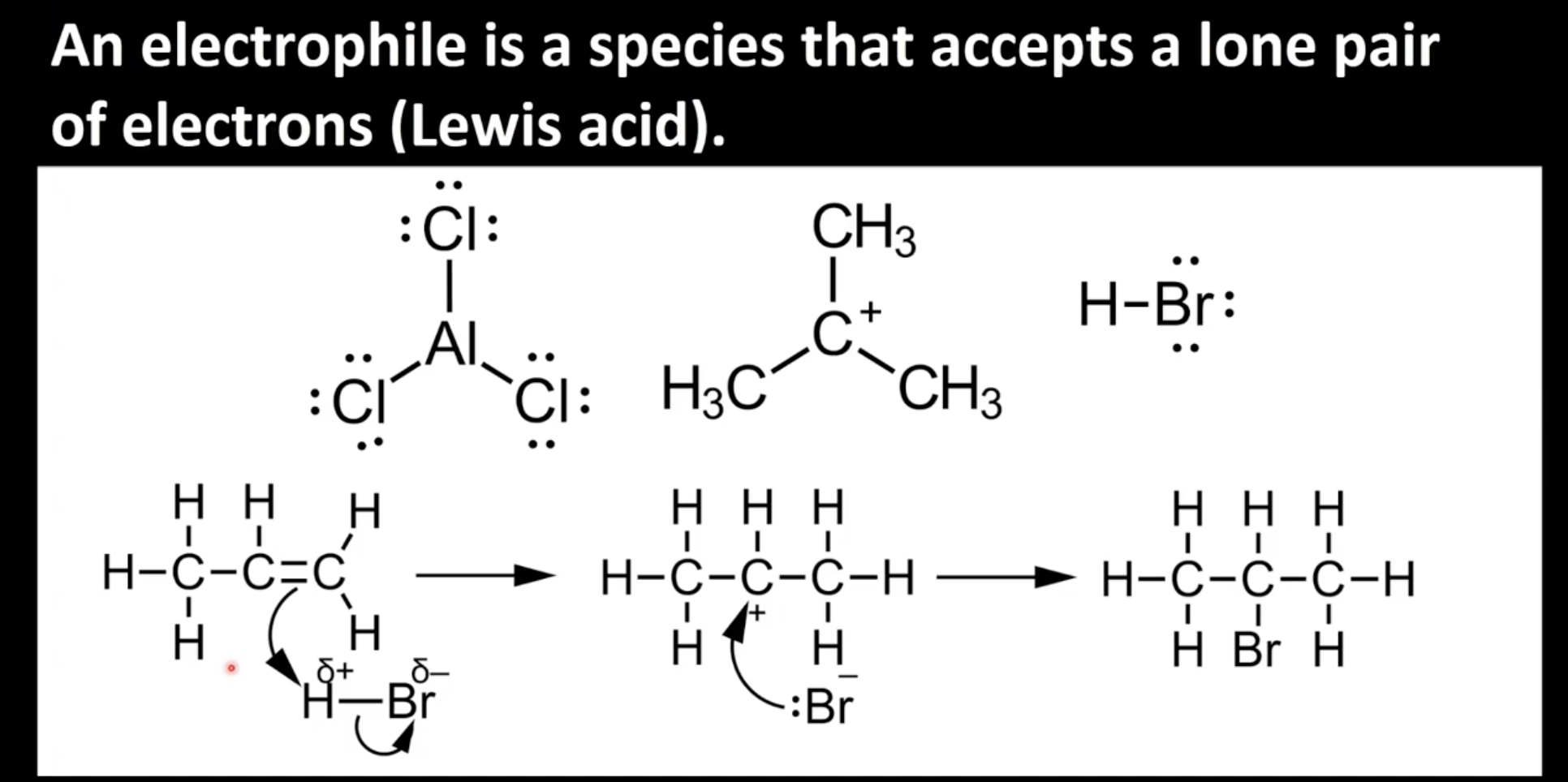
5
New cards
Nucleophiles
Electron rich species that donate lone pairs of electrons
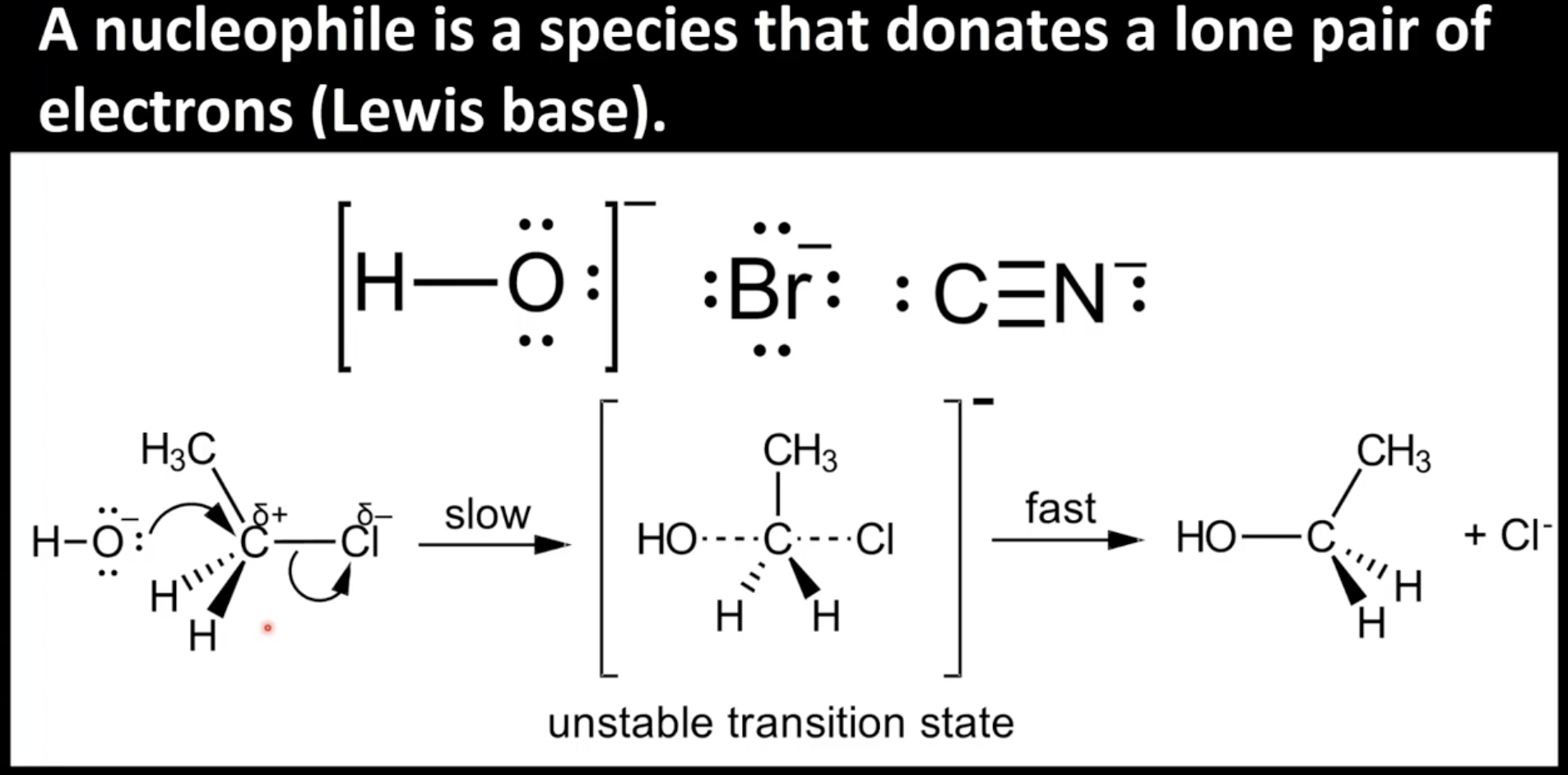
6
New cards
What are Lewis acids and bases in terms of complex ions?
Lewis acid: Central metal ion
Lewis base: Ligand