Chapter 15: Benzene and Aromatic Compounds - Key Terms and Reactions
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
89 Terms
drugs commonly contain
at least one aromatic ring
ex) lipitor
naming monosubstituted benzenes
- "benzene" is parent name
- does not need a number for the substituent
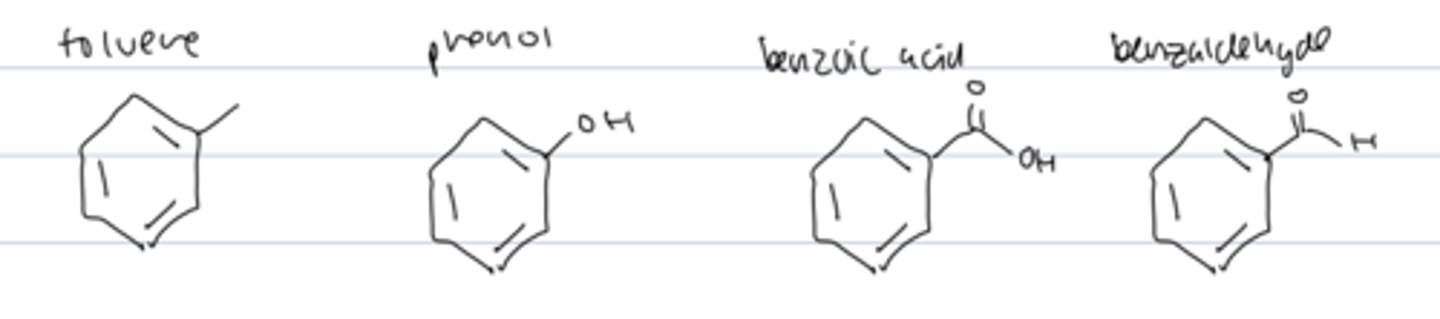
common names for benzene derivatives accepted by the IUPAC
toluene
phenol
benzoic acid
benzaldehyde
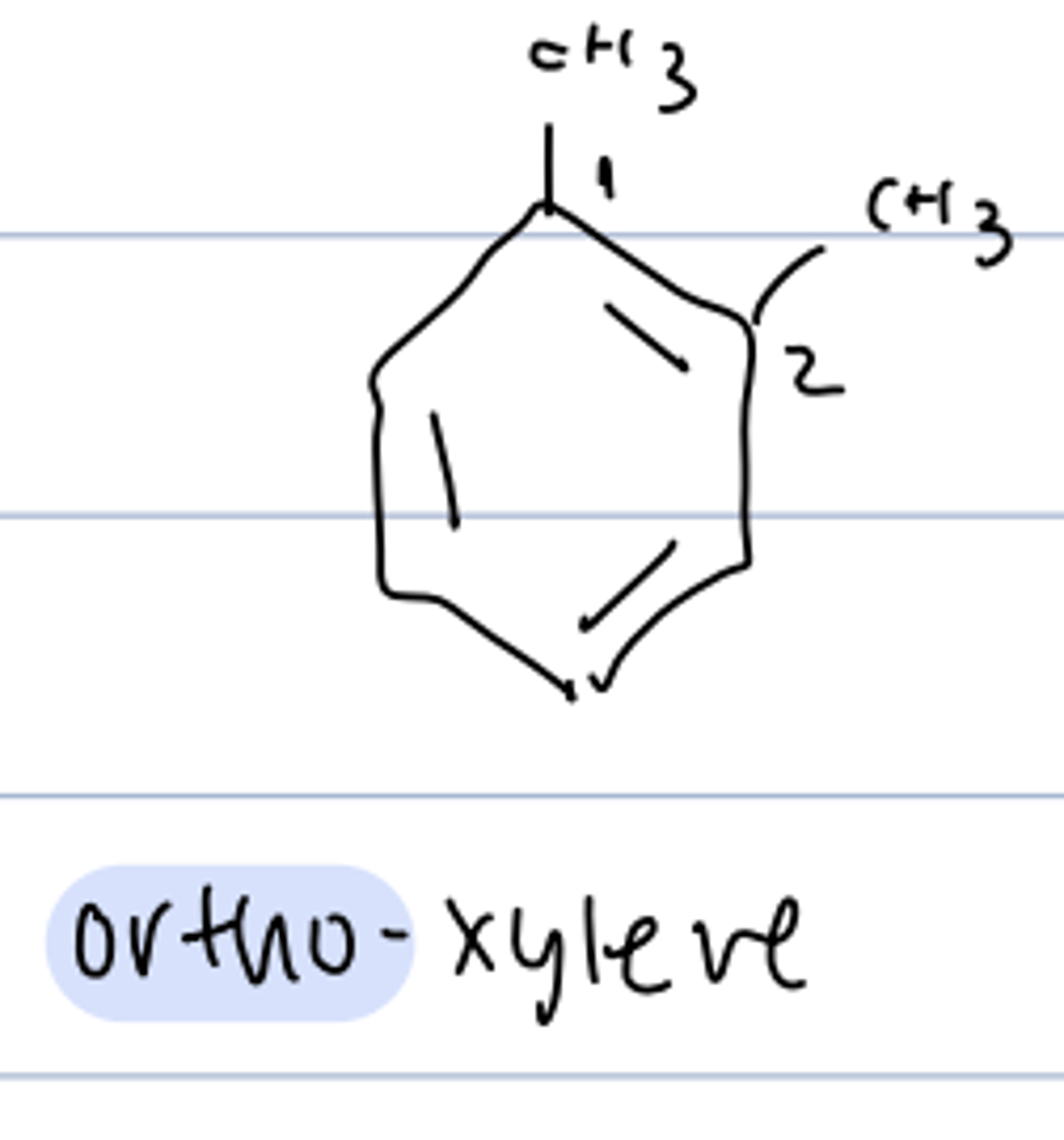
naming disubstituted benzenes
- use parent name "xylene
- show different HNMRs
- ortho, meta, or para indicate the relative location of the substituents

ortho-xylene
1,2-dimethylbenzene
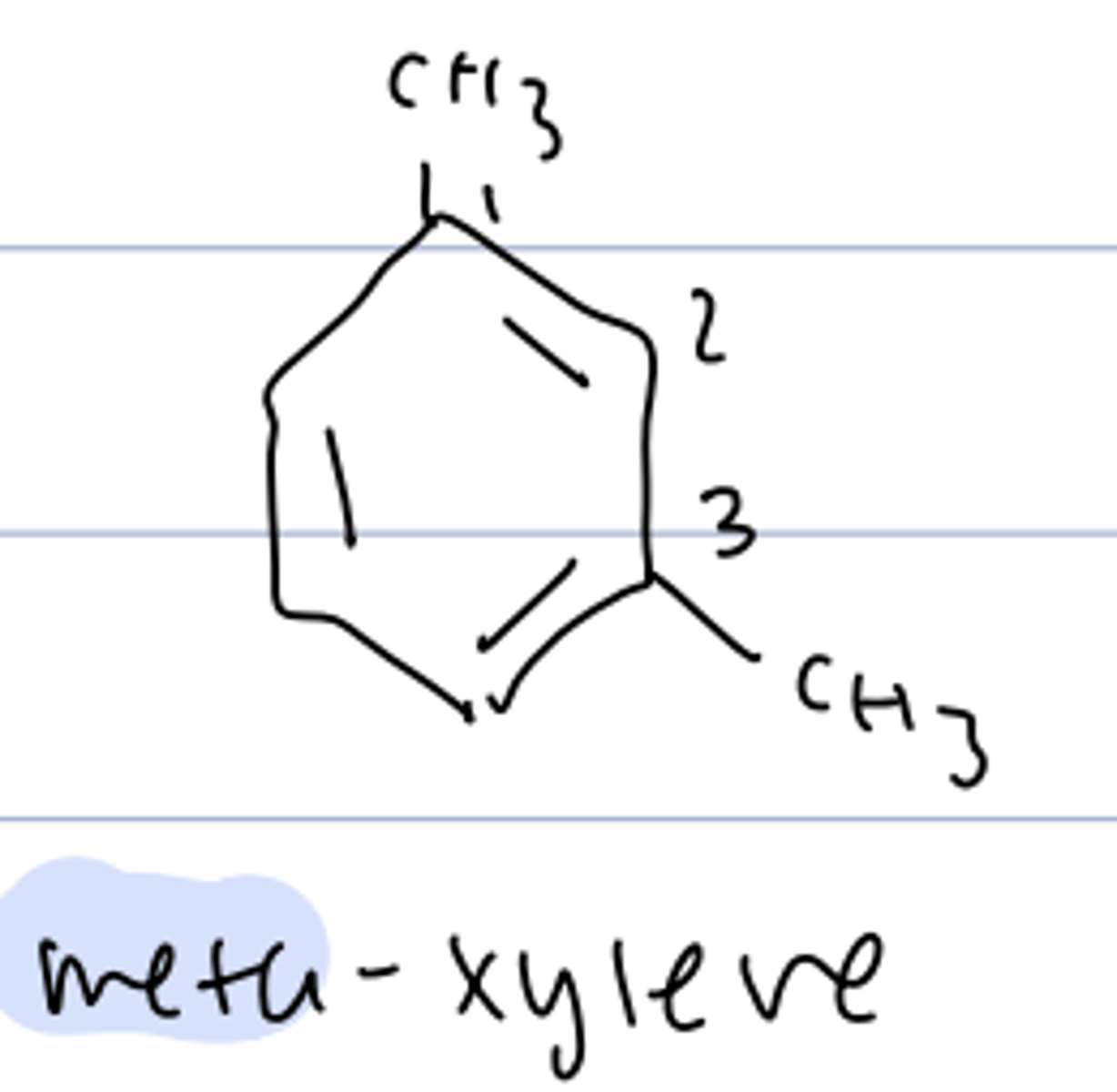
meta-xylene
1,3-dimethylbenzene
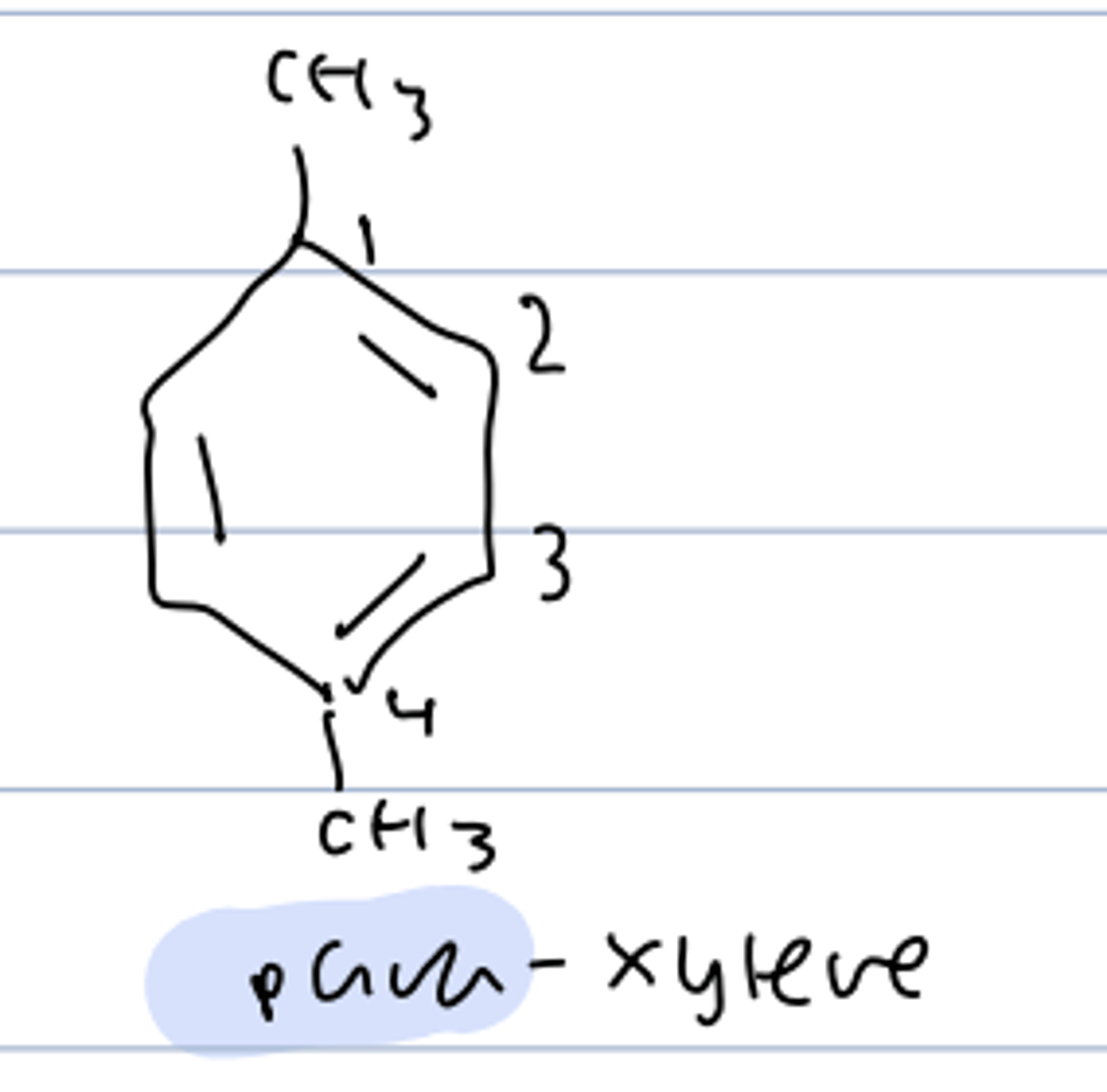
para-xylene
1,4-dimethylbenzene
substituted benzenes
arene
naming substituted benzenes
1) identify the parent stem, such as "benzene," "phenol," "aniline," etc.
2) name all substituents.
3) number the stem carbons.
4) write the name with the substituents arranged in alphabetical order, each preceded by its locant.
benzene aromaticity
- aromatic compounds associated with fragrant aromas
ex) cinnamaldehyde and vanillan
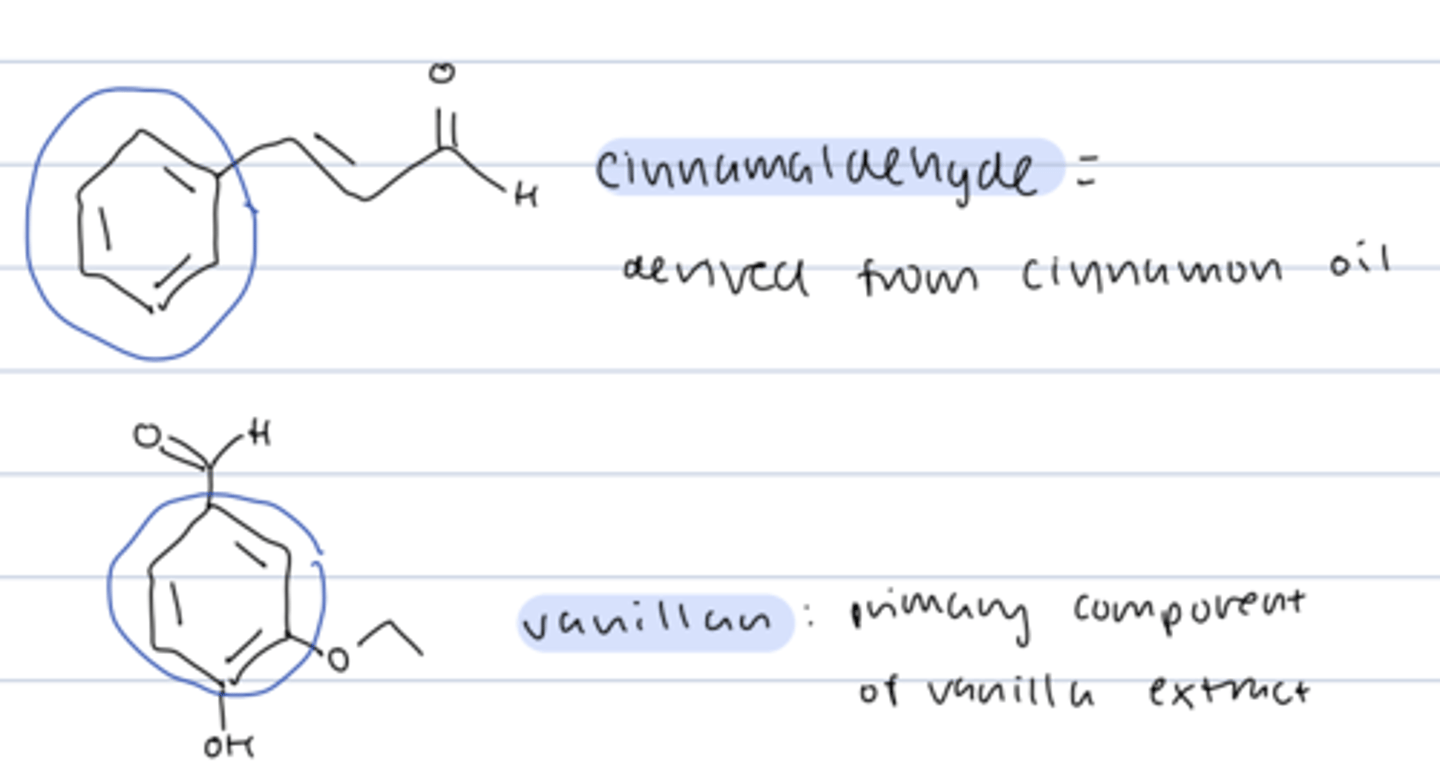
fragrant compounds from natural sources had unexpected properties
- low H:C ratio
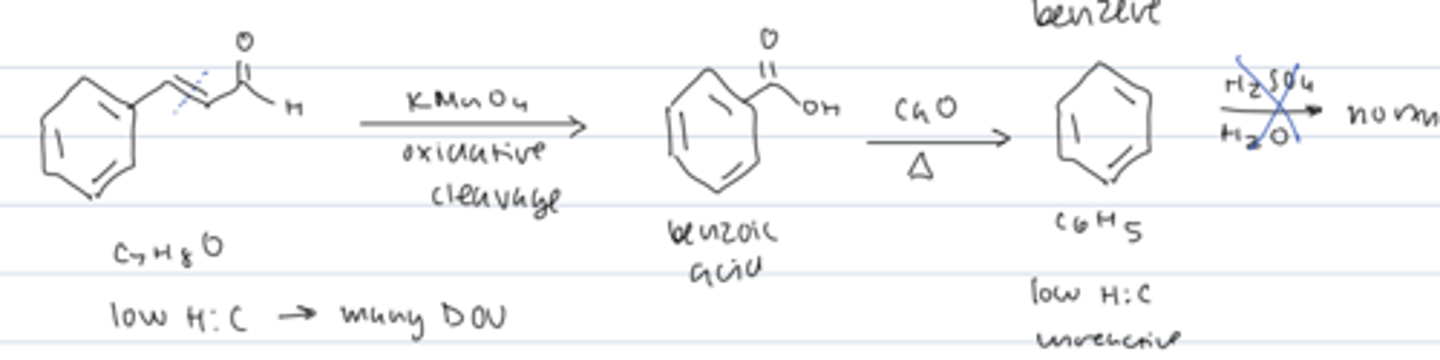
benzene
C6H6
low H:C
unreactive
discovery about benzene in the 1930's
planar with equal C-C bond lengths (x-ray diffraction)
structure of benzene
- all C's are sp2 hybridized
- unhybridized p orbitals
- continuous p orbital overlap

resonance of benzene
- 2 resonance structures with equal contribution to hybrid
- large delocalization energy

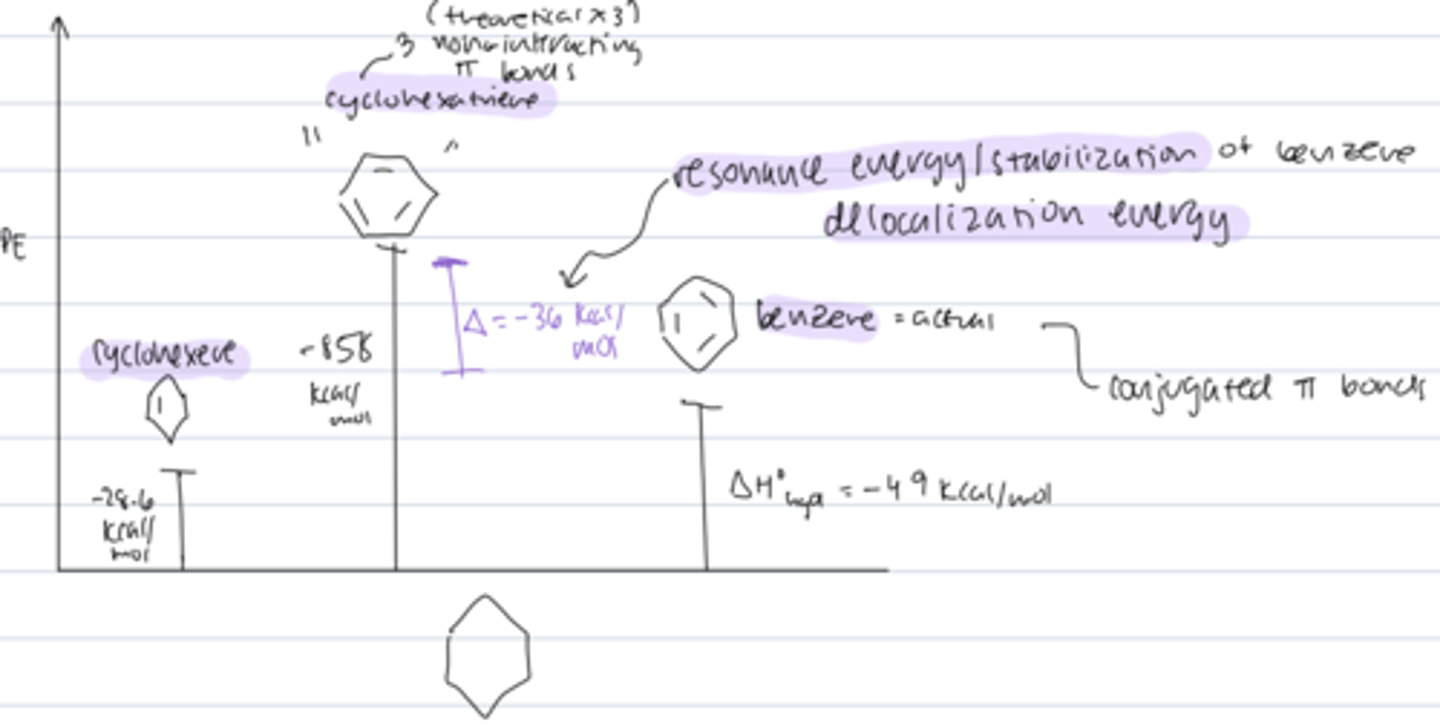
heat of hydrogenation of benzene reveals
resonance energy/delocalization energy
- cyclohexatriene (theoretical noninteracting 3 pi bonds) should have a ΔHhyd 3x as large as cyclohexene
- actual ΔHhyd of benzene is lower by -36 kcal/mol at -49 kcal/mol
aromatic compounds are
- cyclic: containing some conjugated π bonds
- every atom contains an unhybridized p orbitals
- planar
- delocalization lowers energy of the system
- Huckel's rule
Huckel's rule
4n+ 2 pi electrons
- n is any integer
- rule in making a structure aromatic
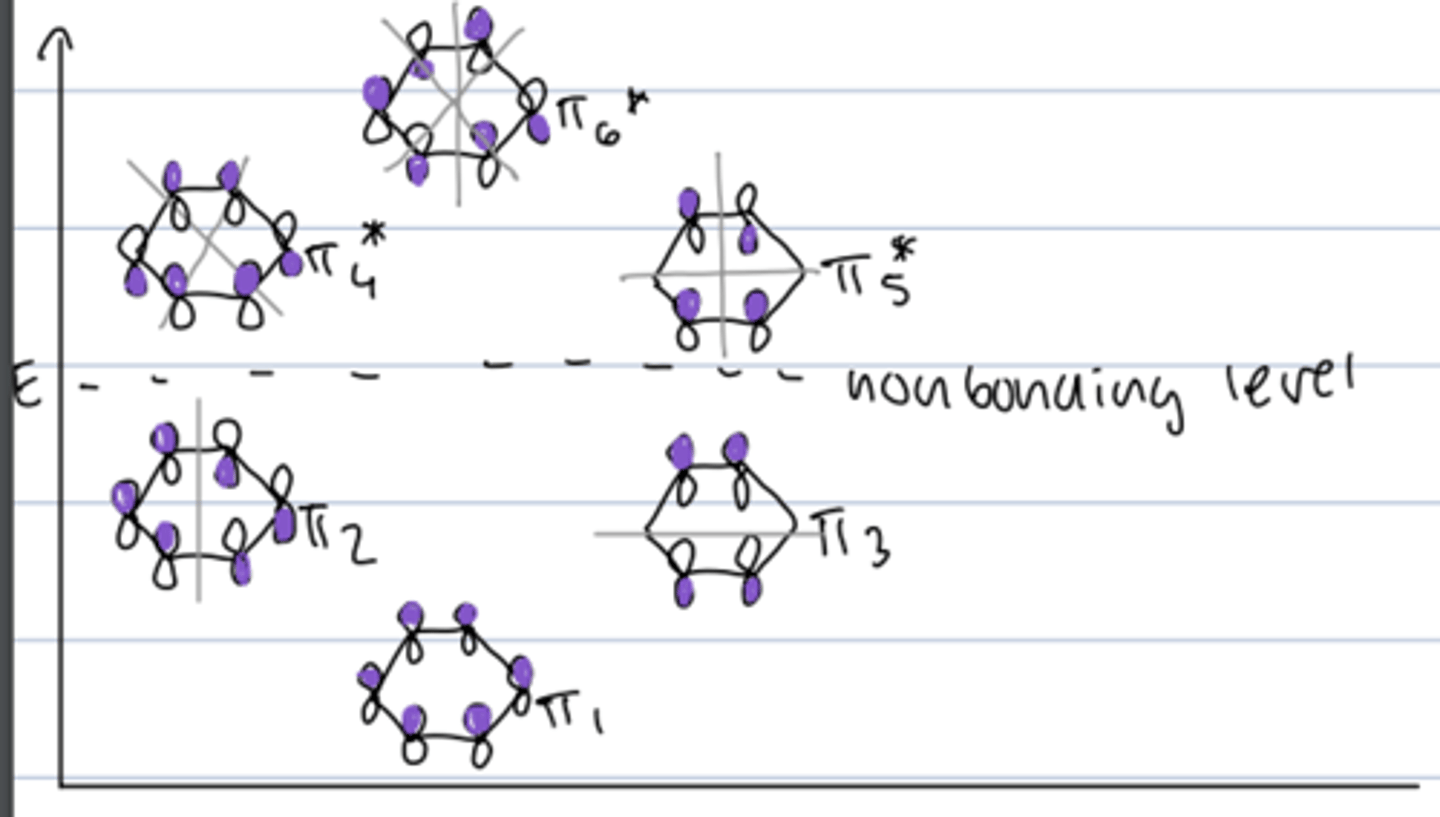
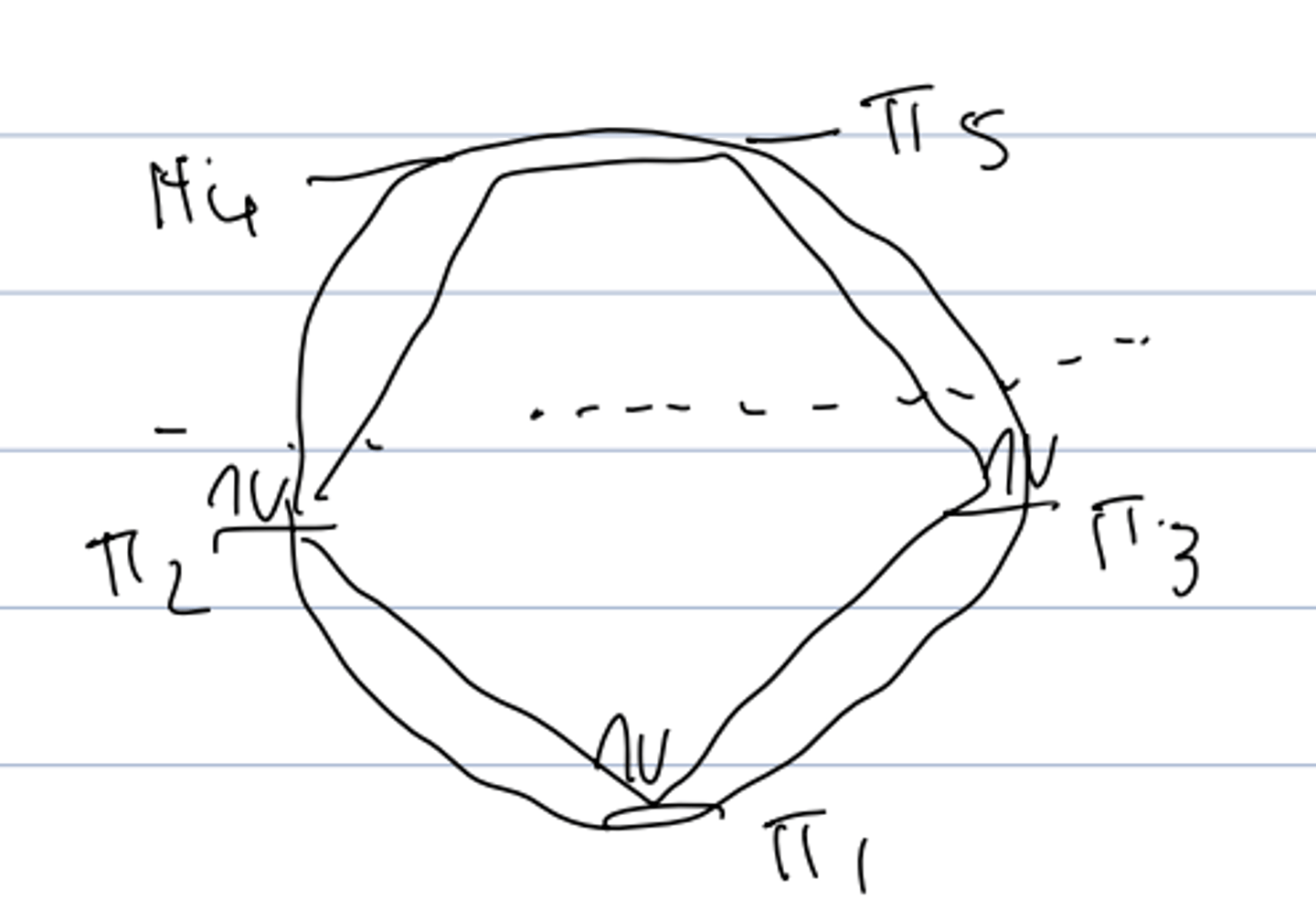
molecular orbital diagram of benzene
- degenerate orbitals: π2 and π3; π4 and π5
- filled bonding orbitals --> closed bonding shell stability
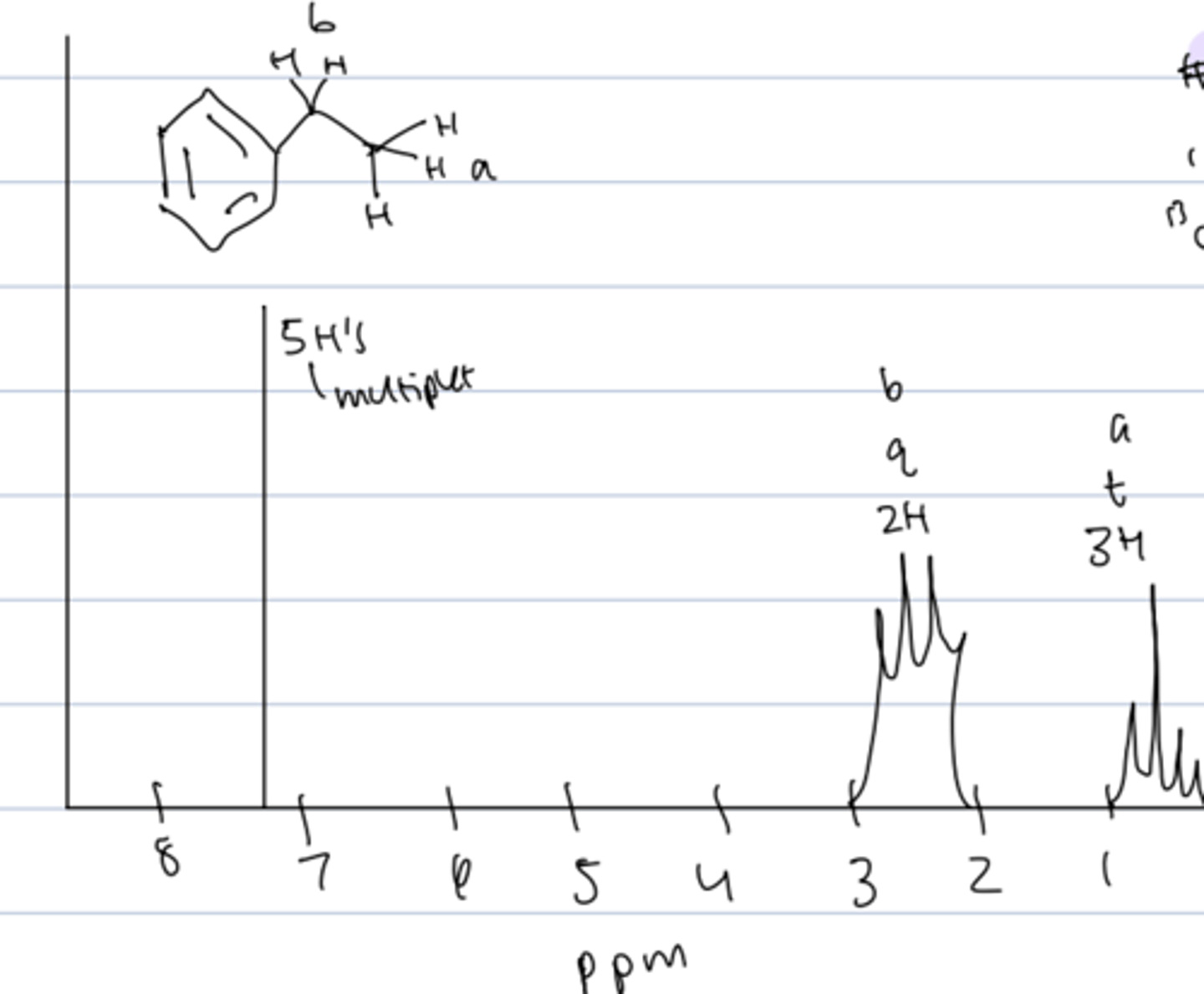
aromatic HNMR
- for benzene: 5 H's multiplet at 7-8 ppm
- H's on C adjacent to ring: 2 H's quartet at 2 to 3 ppm
- H's on terminal methyl group of ethyl substituent: 3 H's triplet at 0-1 ppm
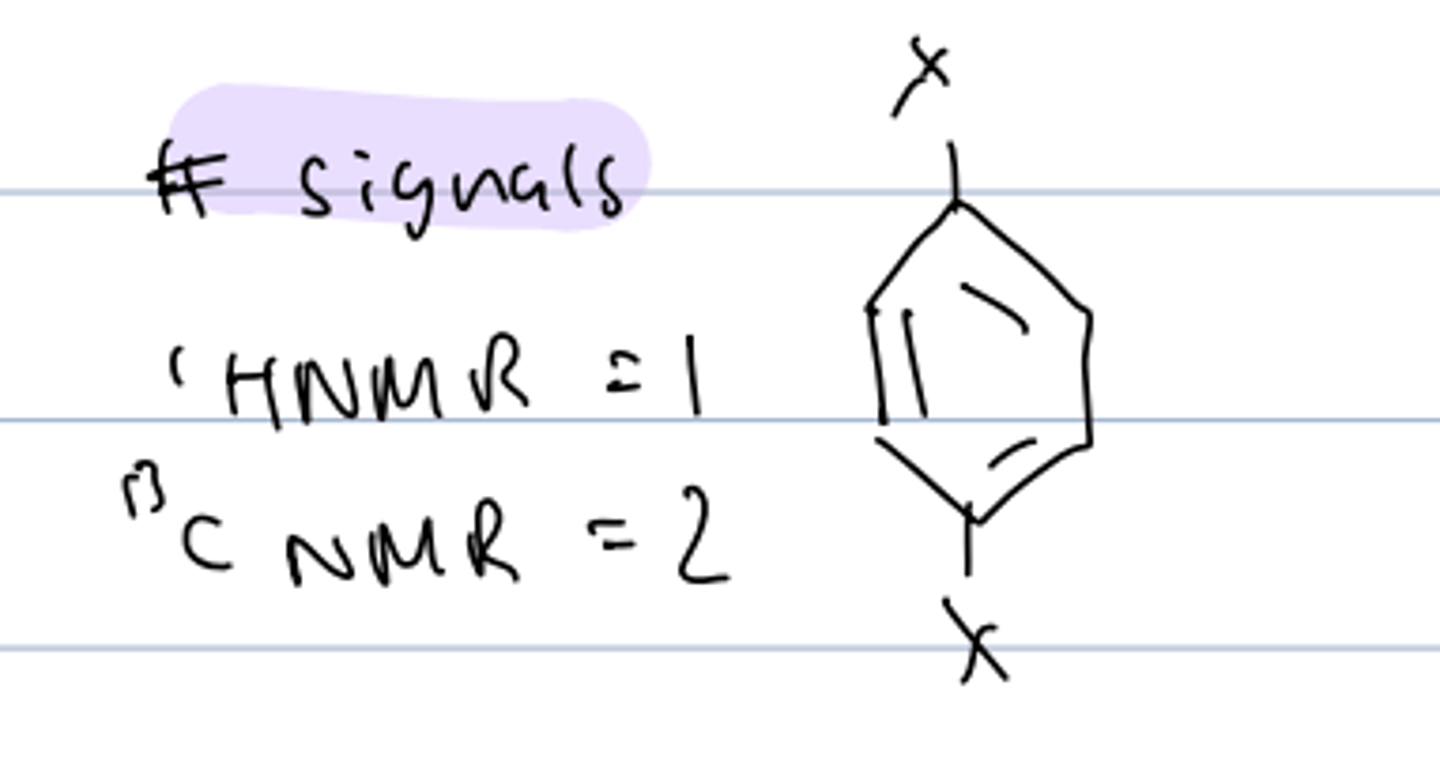
# signals of same substituent, disubstitued para-benzene
HNMR: 1
CNMR: 2
# signals of diff substituent, disubstitued para-benzene
HNMR: 2 dd
CNMR: 4
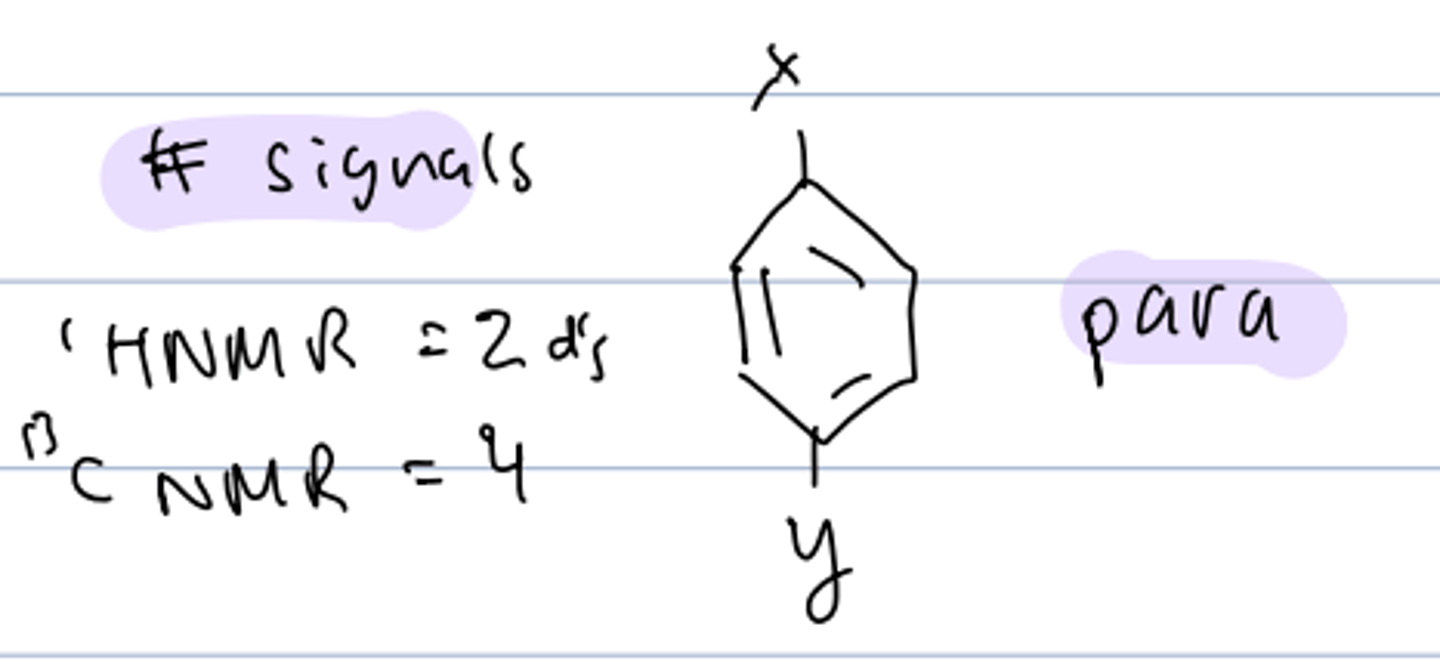
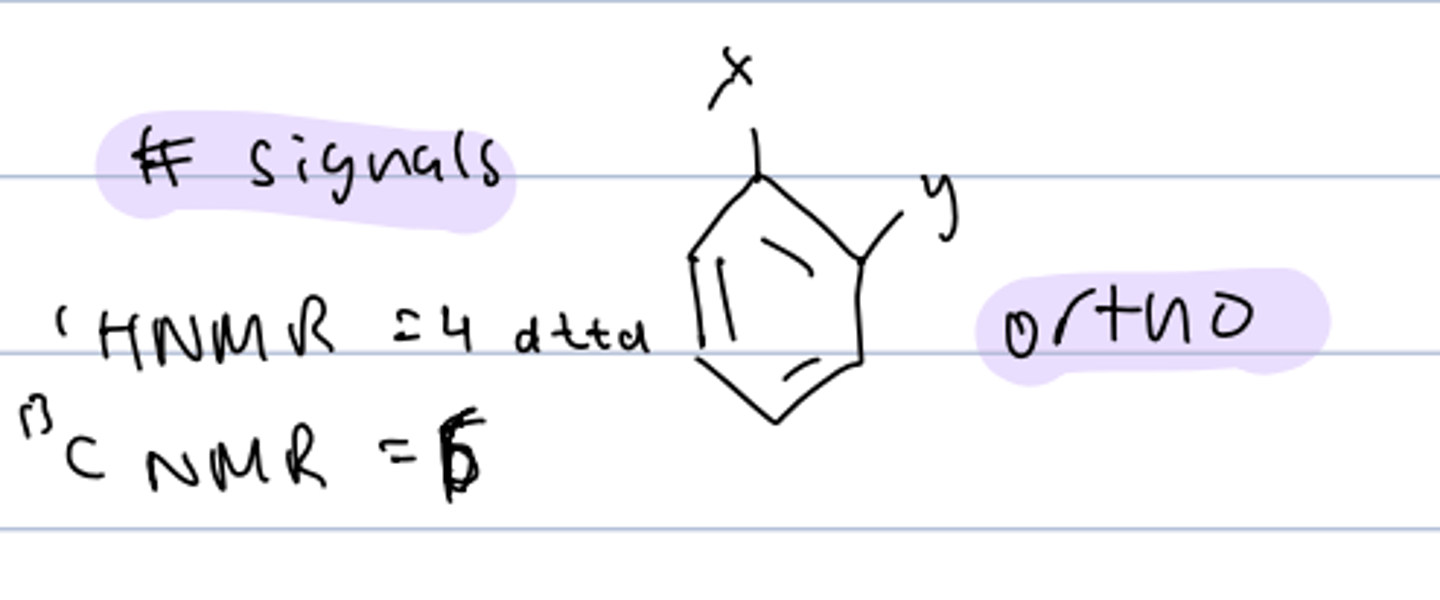
# signals of diff substituent, disubstitued ortho-benzene
HNMR: 4 dttd
CNMR: 6
# signals of diff substituent, disubstitued meta-benzene
HNMR: 4
CNMR: 6
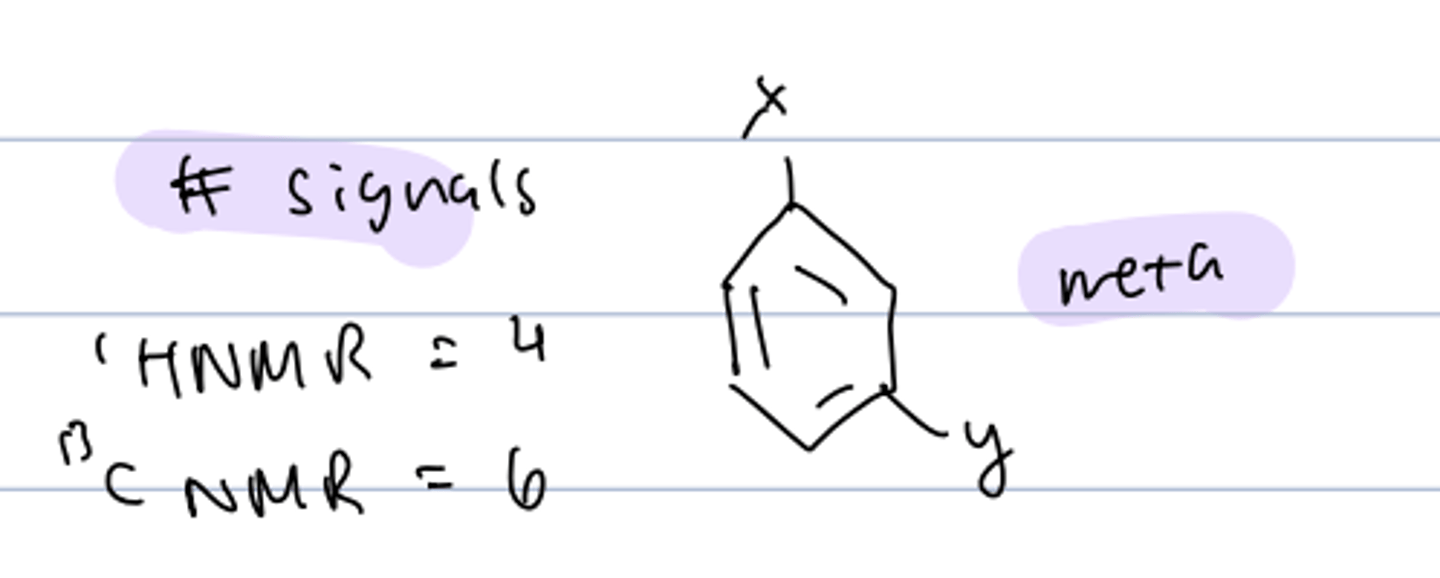
anti-aromatic compounds
- cyclic: containing some conjugated π bonds
- every atom contains an unhybridized p orbitals
- planar
- delocalization increases E
- 4n π electrons, n=integer
cyclobutadiene
- antiaromatic (4 π e-)
- very reactive
reactivity of cyclobutadiene
diels-alder reaction with itself rapidly (hard to isolate it at room temperature) yields mix of endo and exo products
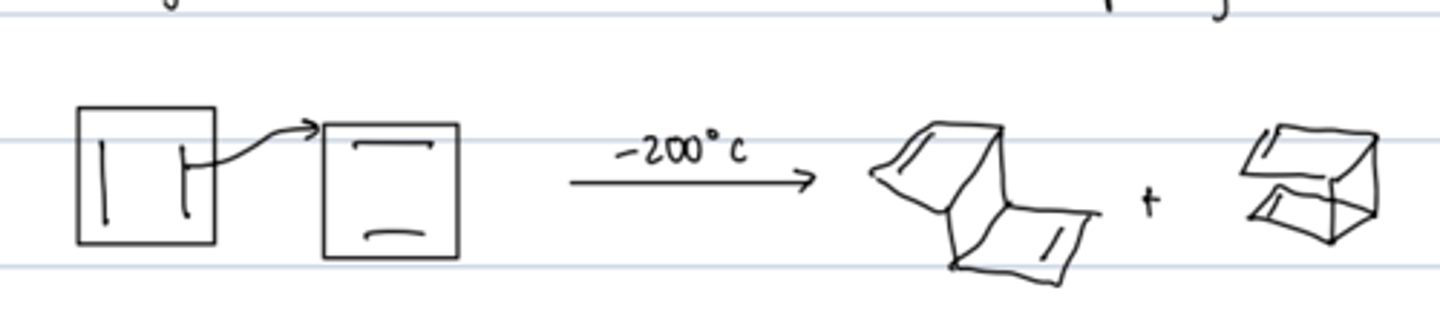
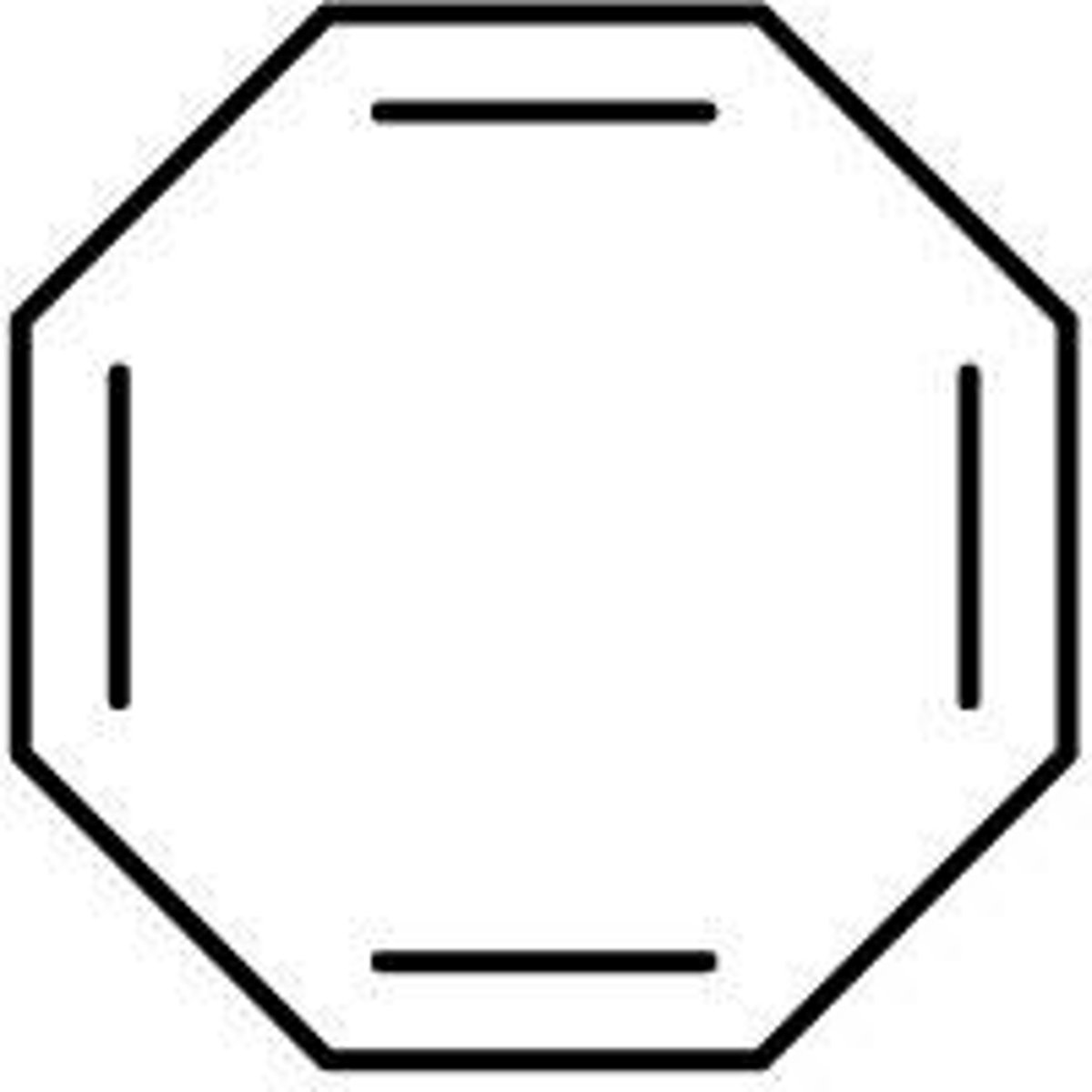
cyclooctatraene
- nonplanar so nonaromatic
- 4n π e-; n=2
bond angle in cyclooctatraene
- ideal: 120º
- in planar: 135º
angular strain (expansion)
shape of cyclooctatraene
to reduce angle strain, it adopts a tub shape
- loses p orbital overlap
- nonplanar
reactivity of cyclooctatraene (non-aromatic)
behaves like typical alkene
nonaromatic compounds
do not have a continuous ring of overlapping p orbitals and may be nonplanar
[n]-annulenes
higher cyclic, conjugated polyenes where n = # of atoms in the ring
example of aromatic annulene
[14]-annulene
- 4n+2 where n=3
- planar and ok bond angles
![<p>[14]-annulene</p><p>- 4n+2 where n=3</p><p>- planar and ok bond angles</p>](https://knowt-user-attachments.s3.amazonaws.com/6de49587-a25e-4a04-8fa9-5b97accf45b4.png)
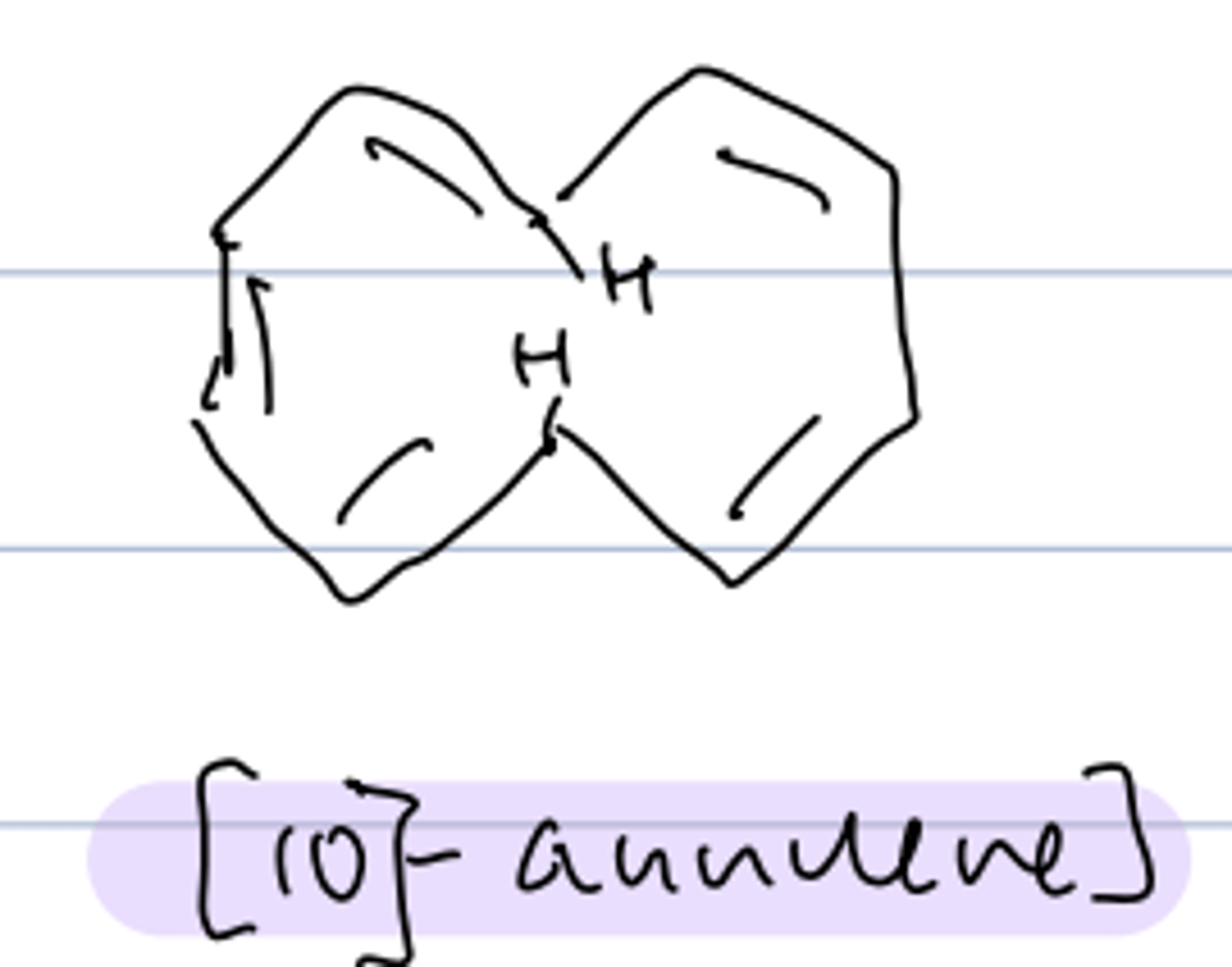
[10]-annulene
- 10 π electrons: 4n+2 --> expect aromatic
- nonplanar shape to avoid steric interference of H's so
non-aromatic
frost circle analysis (inscribed polygon method)
represents MO's and can show reactivity for planar, conjugated cyclic systems
- draw the ring inside a circle with a point at the bottom the circle where each point of ring touches the circle
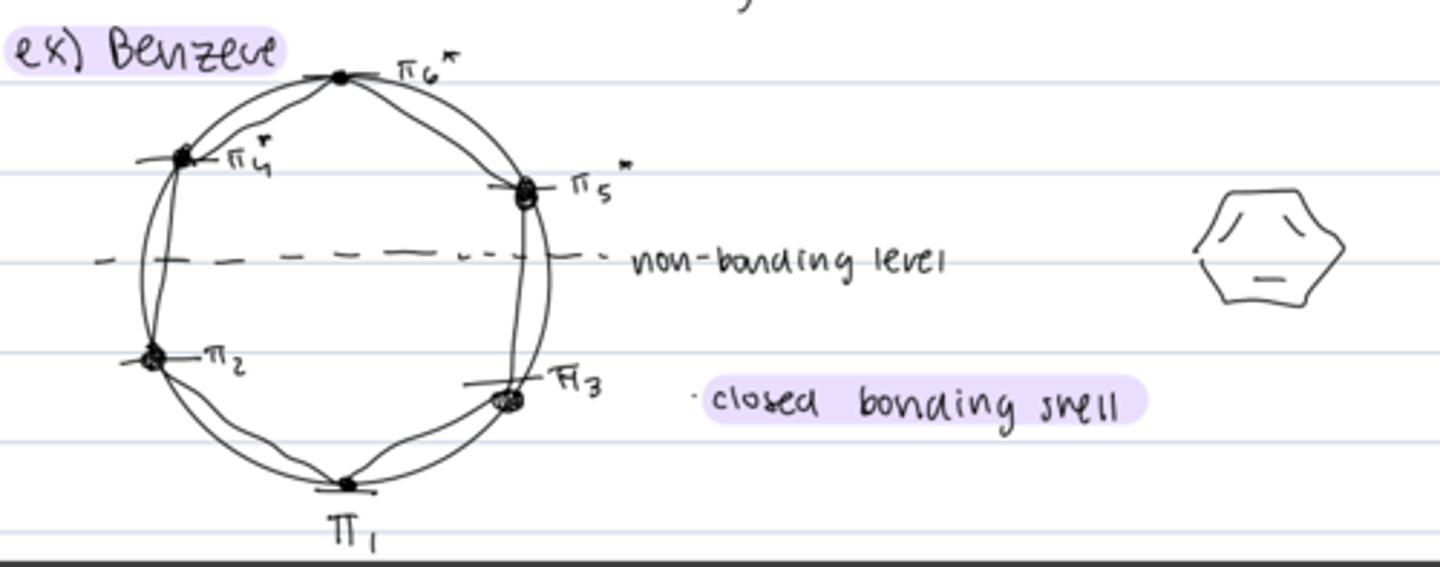
frost circle analysis of benzene
shows closed bonding shell --> explains stability
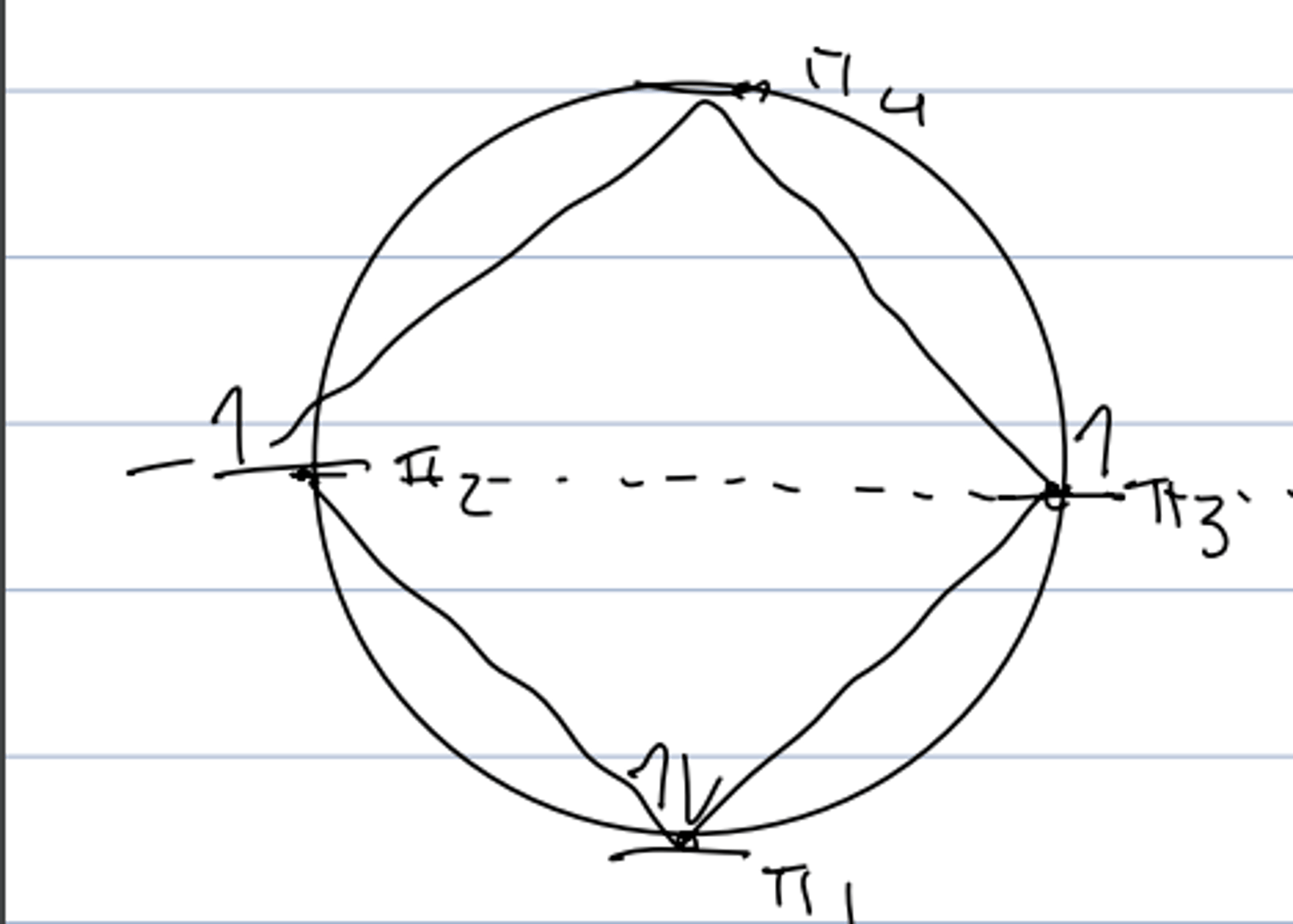
frost circle analysis of cyclobutadiene
- shows unpaired electrons: diradical character --> very reactive
aromatic ions
huckel's rule applies if charge can be delocalized around the ring
cyclopentadiene
-conjugate base is aromatic
-planar conjugated system with 6 pi electrons
-produces stable free radical
- pKa = 16
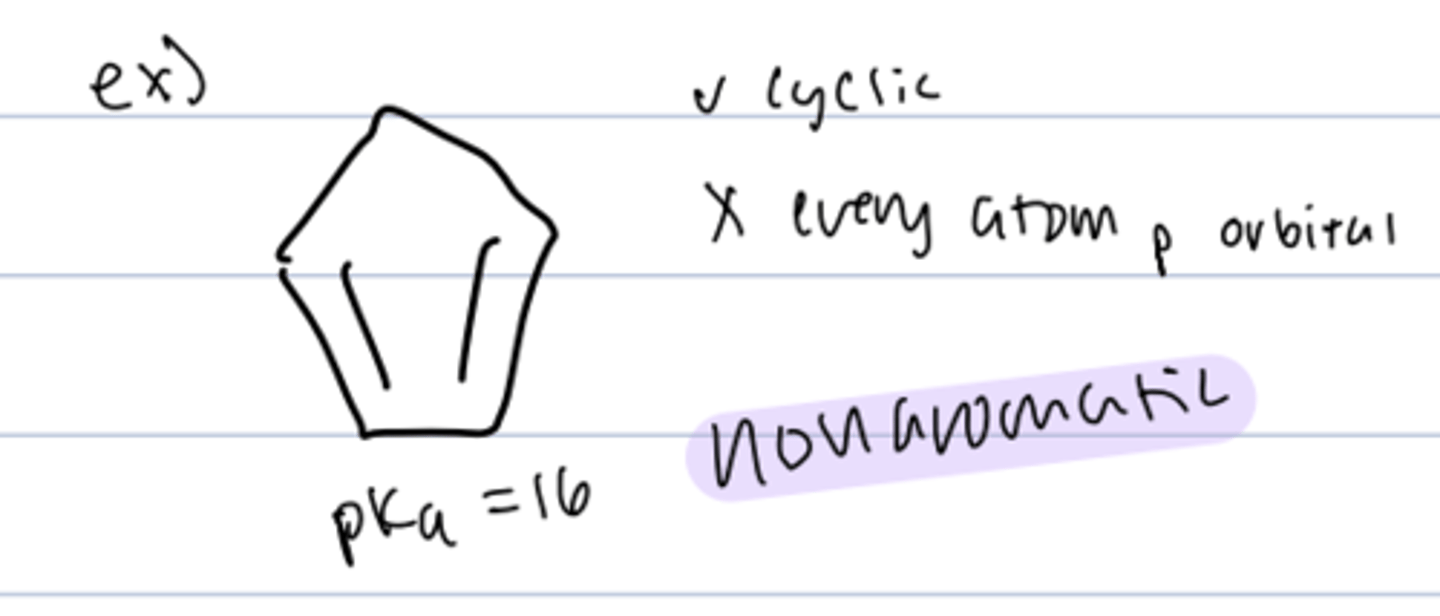
cyclopentadiene to form cyclopentadienyl anion
- deprotonate with a base
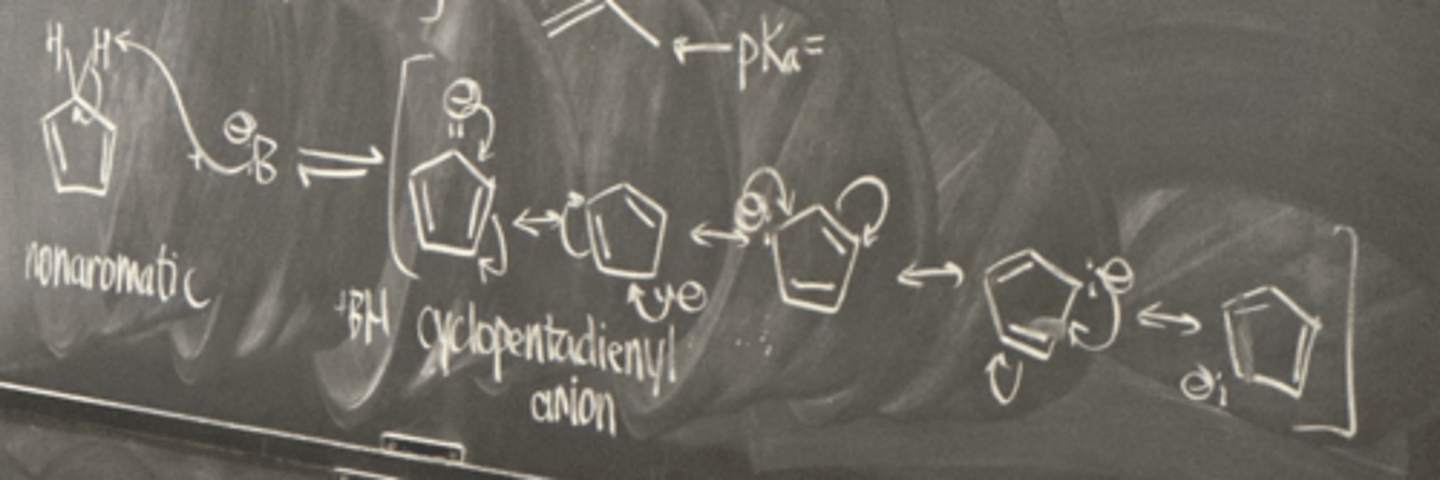
cyclopentadienyl anion
aromatic anion: closed bonding orbital
- 5 resonance structures
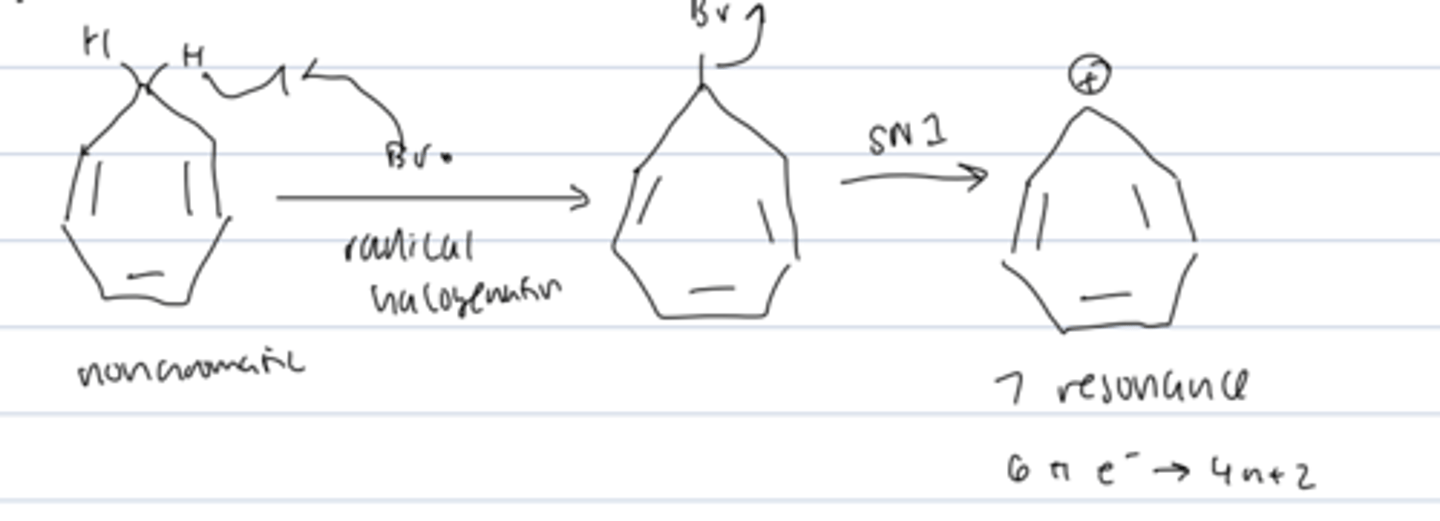
cycloheptatrienyl cation
formed from the nonaromatic cycloheptatriene by radical halogenation followed by SN2
- stabilized by 7 resonance forms
- aromatic: 6 π electrons
1,3,5,7-cyclooctatetraene
- nonplanar
- nonaromatic
how to turn 1,3,5,7-cyclooctatetraene into aromatic?
treat with 2 K⁰ - 2 electron reduction
- produces a planar that is delocalized by resonance
- also produces 2 K+
2π electrons (4n+2, n=0)
aromatic
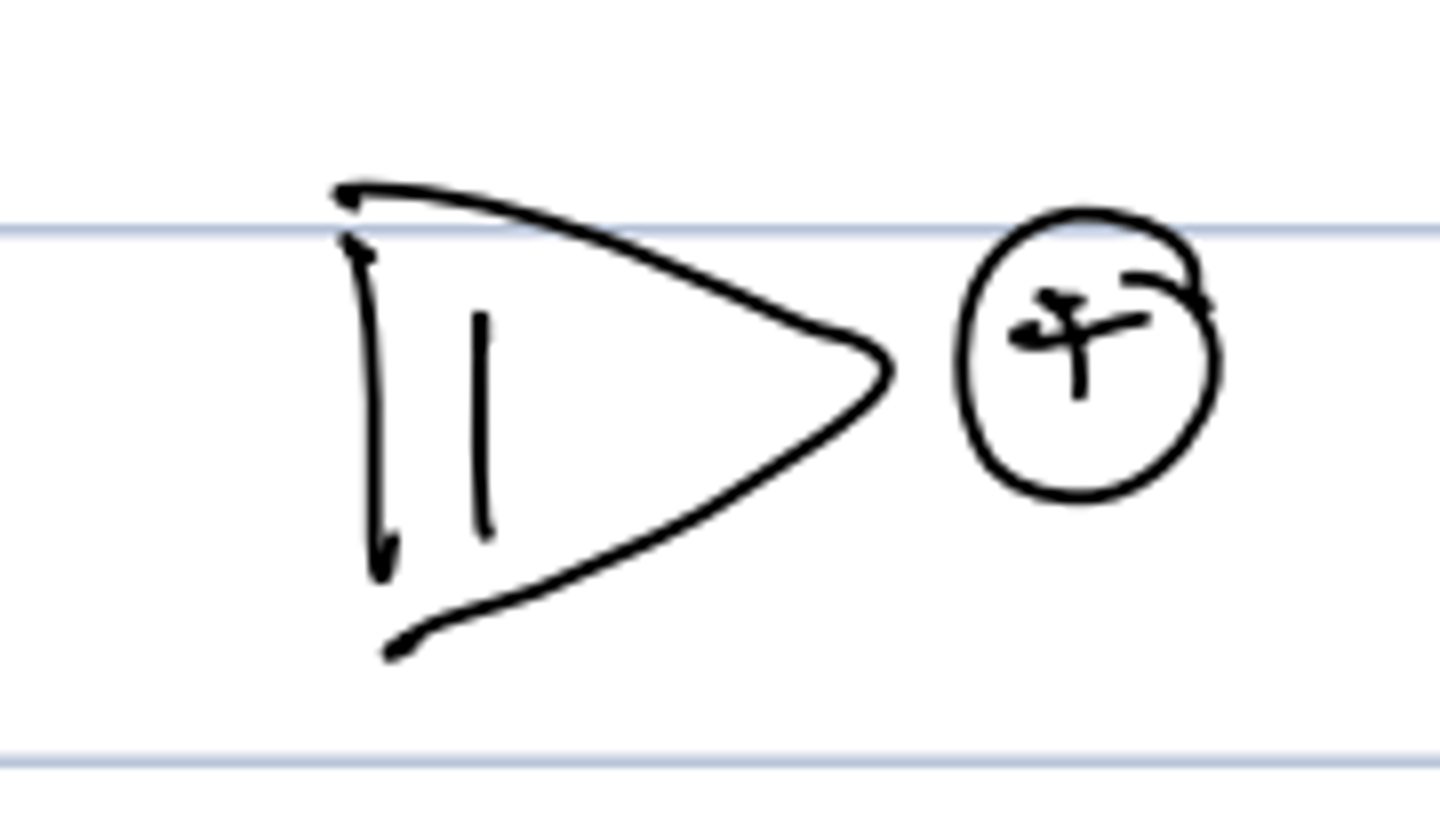
4π electrons
anti-aromatic
reactive
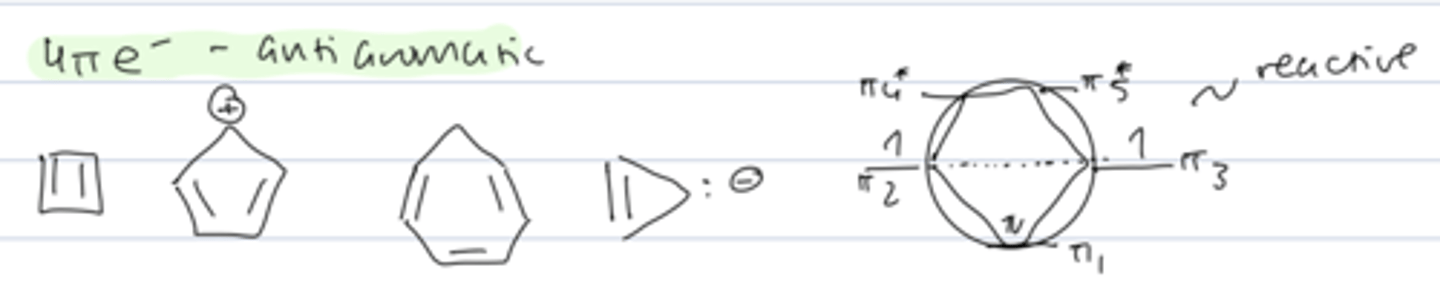
6π electrons
aromatic
heterocyclic aromatic compounds
aromatic compounds that contain at least one atom other than carbon within the ring
ex) pyridine and imidazole
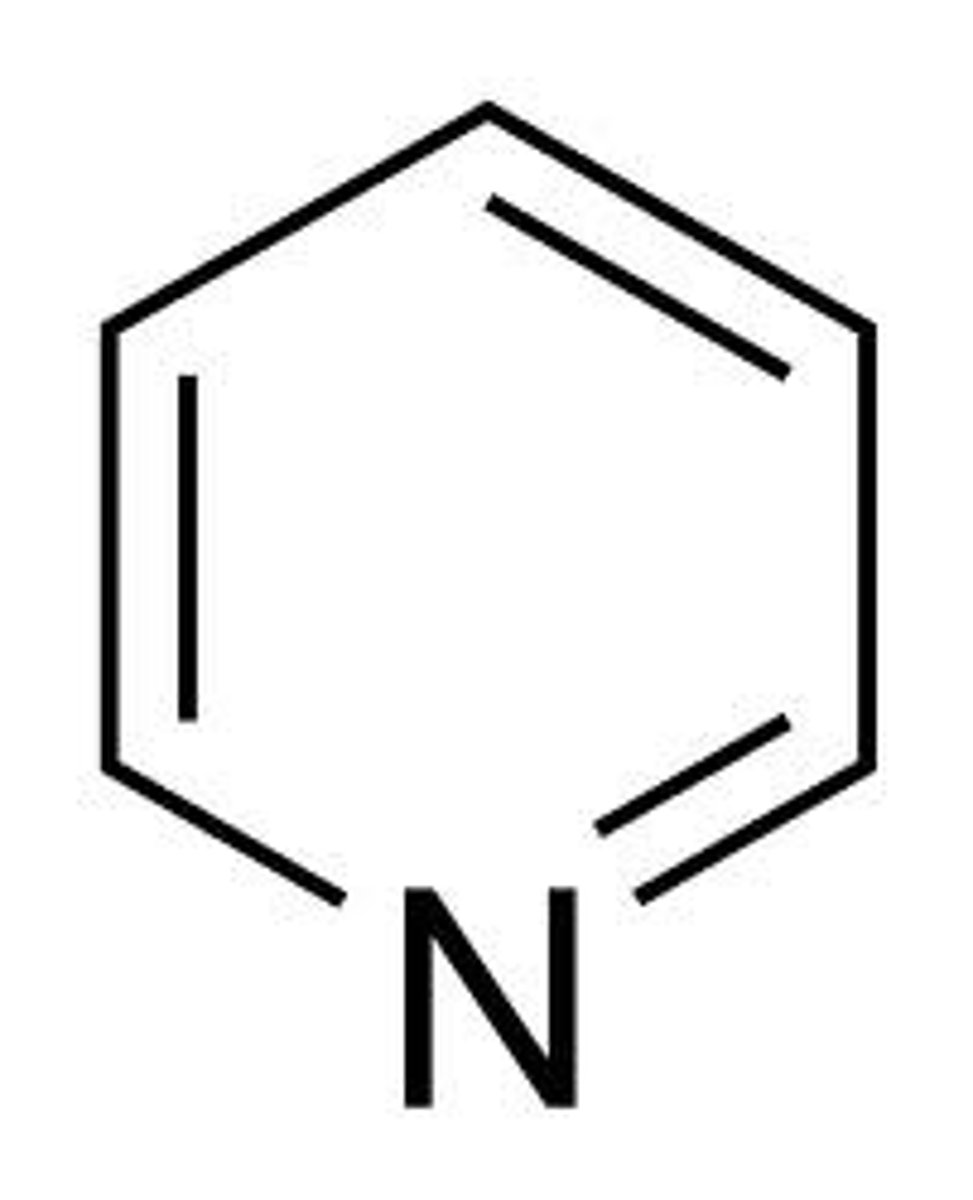
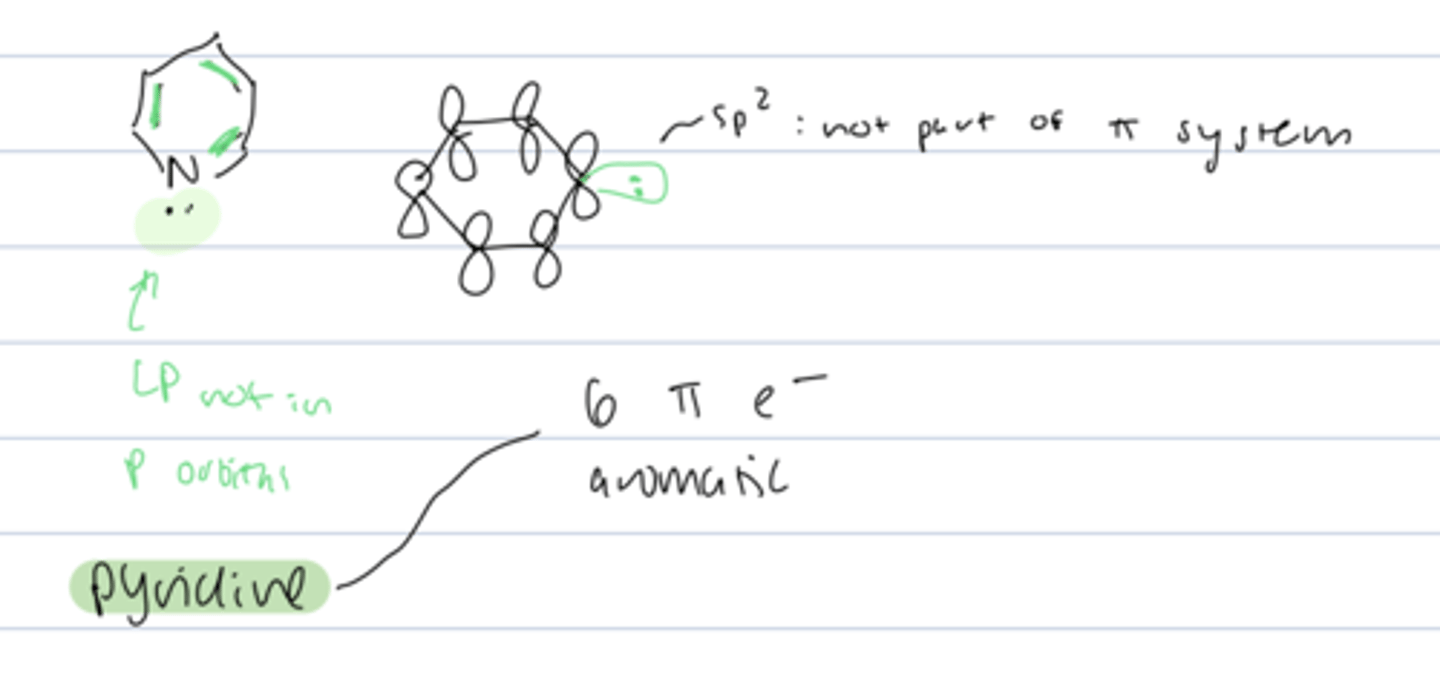
pyridine
- 6 π electrons = aromatic
- LP is not part of π system bc sp² hybridized
imidazole
- 6 π electrons = aromatic
- LP that can be delocalized in part of π system
- LP not in π system (sp² hybridized) is more basic

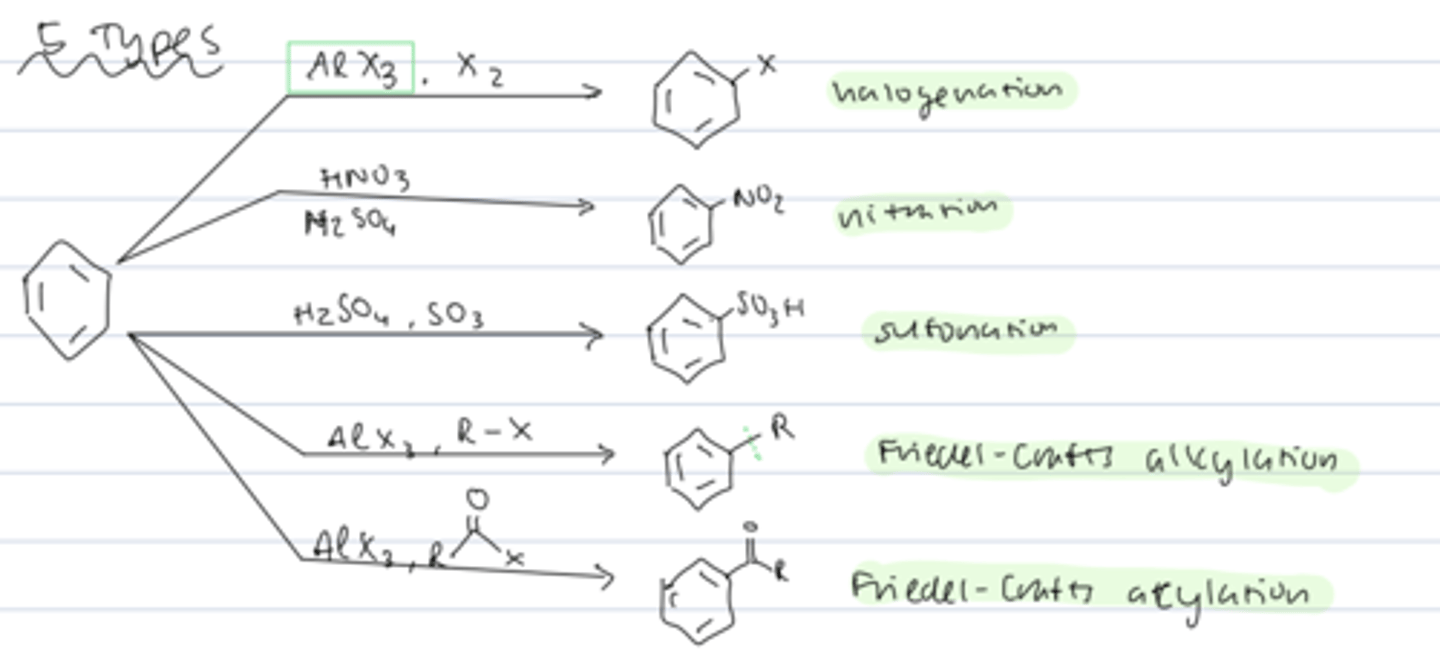
electrophilic aromatic substitution
reaction of benzene with EX to substitute a hydrogen for X
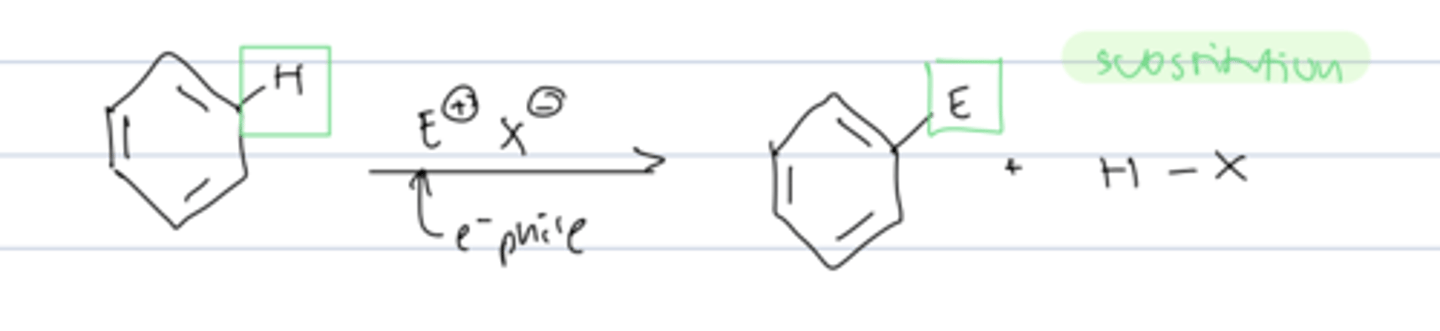
5 types of electrophilic aromatic substitution
halogenation
nitration
sulfonation
Friedel-Crafts alkylation
Fridel-Crafts acylation
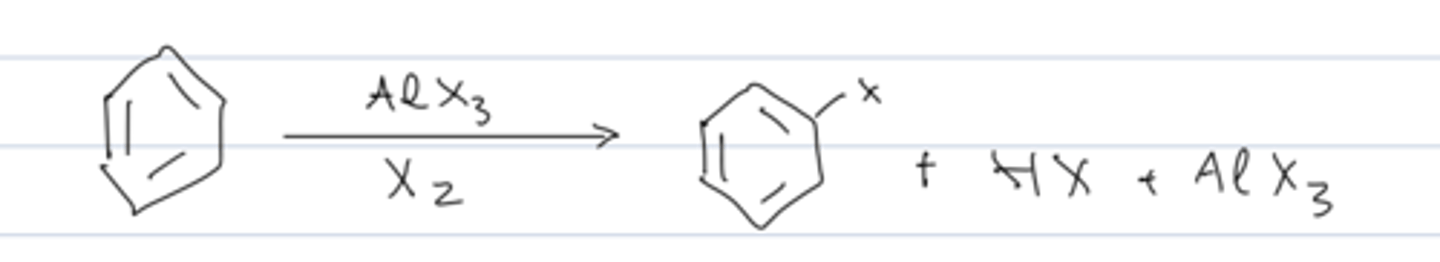
overall reaction of aromatic halogenation
benzene + AlX₃ + X₂ → X-substituted benzene + HX + AlX₃
reagents of aromatic halogenation
X₂ = Cl₂, Br₂
lewis acid catalyst = AlX₃, FeX₃; X matches the halogen
halogenation of benzene becomes more exothermic as
we proceed from I₂ (endothermic) to F₂ (exothermic and explosive)
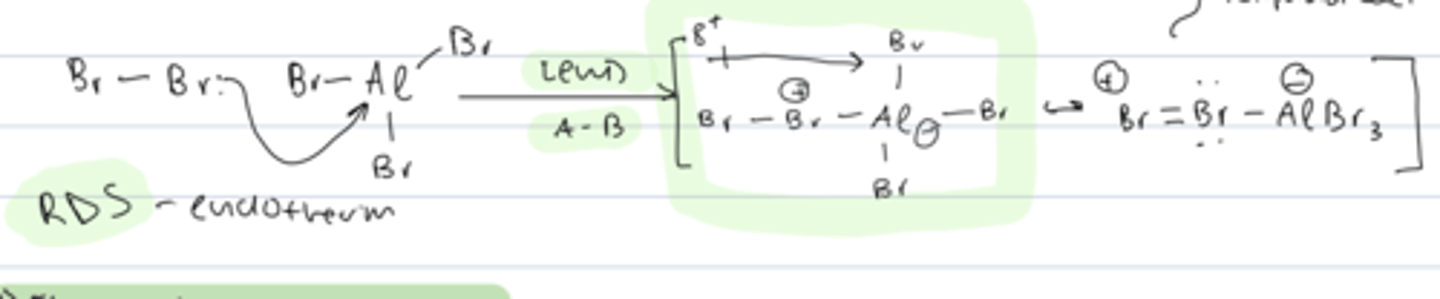
step 1 of aromatic halogenation
activation of electrophile
- RDS
- LP from Br₂ undergoes lewis acid-base reaction with AlBr₃ to form an electrophile
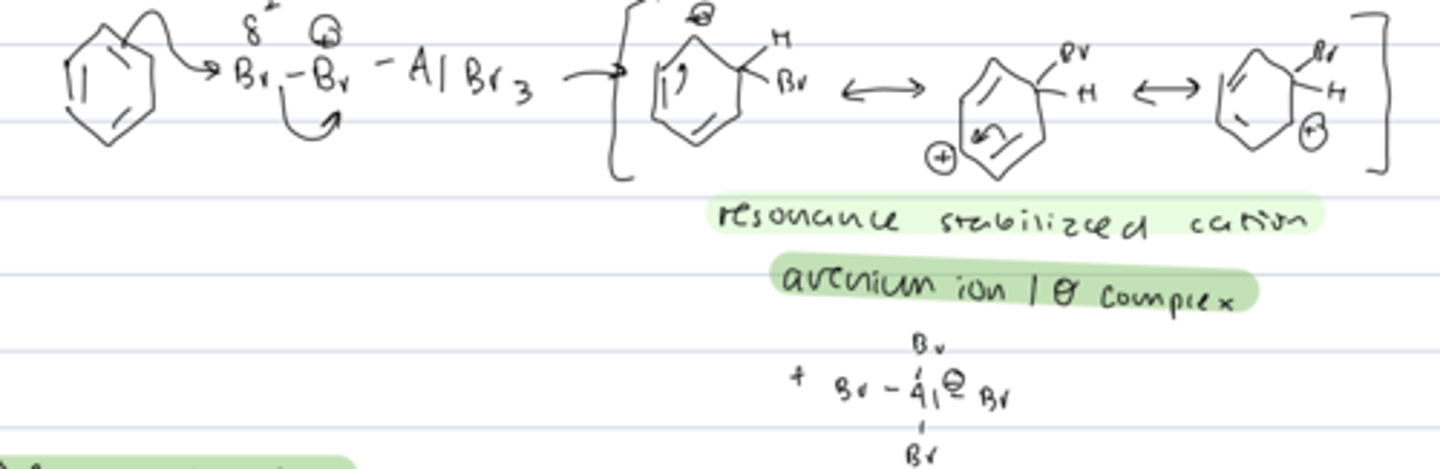
step 2 of aromatic halogenation
electrophilic attachment
- pi bond attacks the partial positive Br and AlBr₄ leaves
- forms a resonance stabilized cation: arenium ion/σ complex
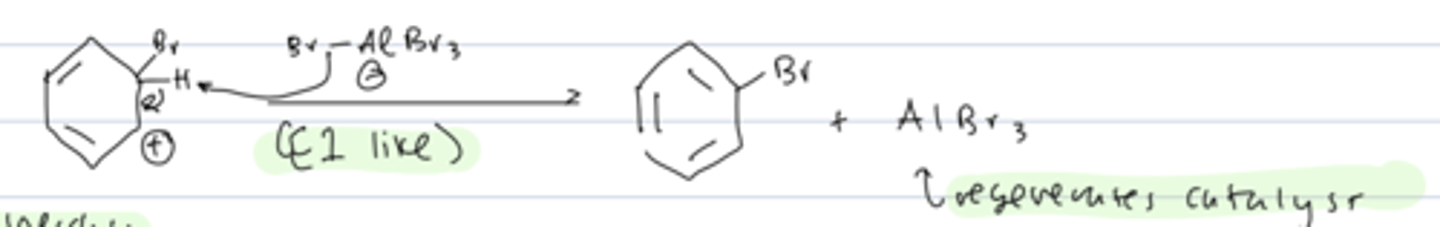
step 3 of aromatic halogenation
rearomatization
- the arenium ion undergoes an E1 like process in which AlBr₄ acts as a base and deprotonates it, with the bond to H transferred to the cation
- regenerates the catalyst and completes substitution
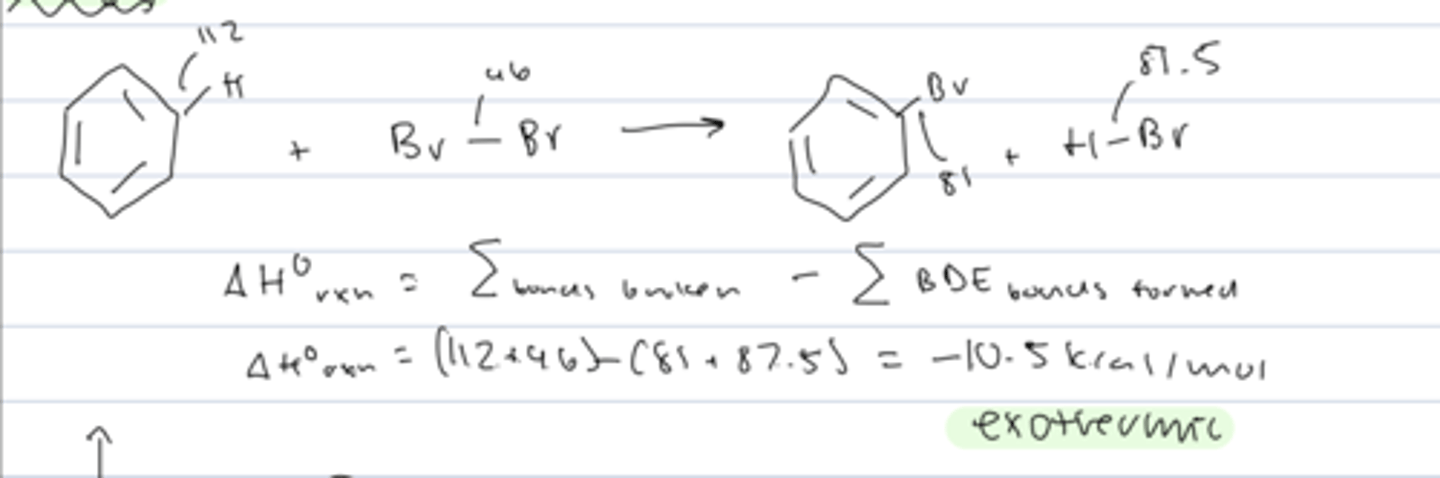
energy of aromatic halogenation
exothermic: bonds broken weaker than bonds formed
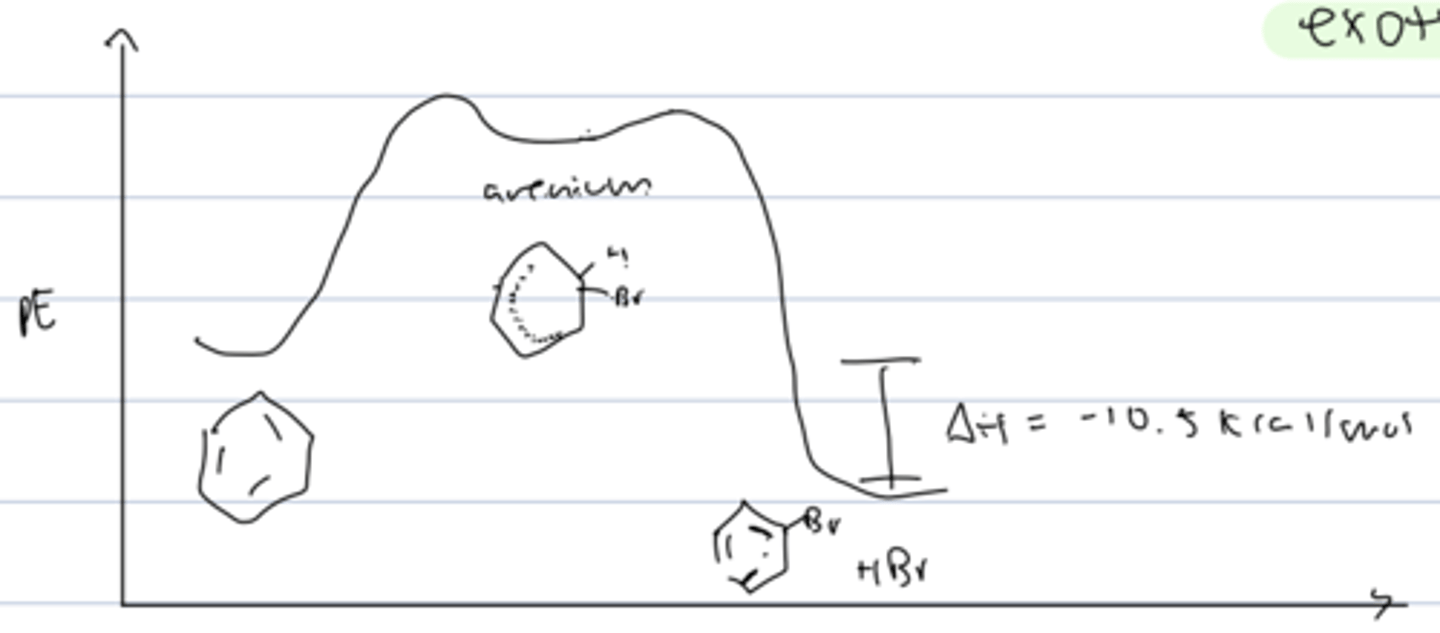
PE diagram of aromatic halogenation
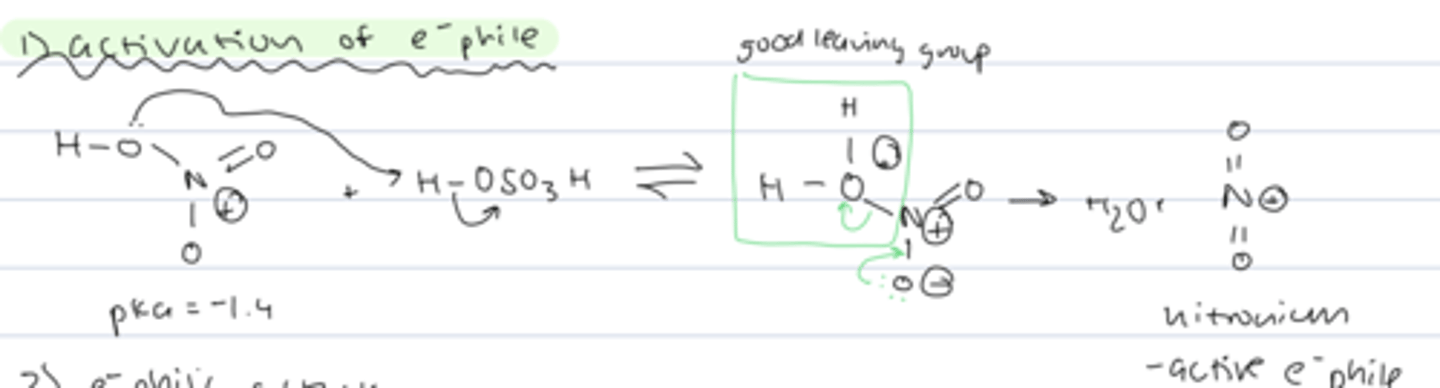
step 1 of aromatic nitration
activation of electrophile
- LP on oxygen of nitric acid deprotonates H₂SO₄
- OH₂+ is a good leaving group, so LP from oxygen can form another N-O double bond and kick out water
- active electrophile = nitronium
step 2 of aromatic nitration
electrophilic attack
- pi bond attacks the positive N of nitronium, with the N-O pi bond migrating to the oxygen
- forms resonance stabilized cation
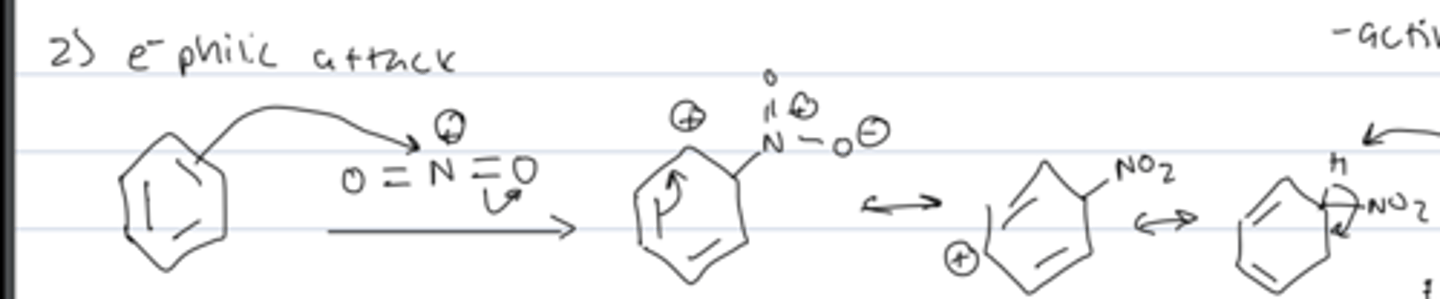
step 3 of aromatic nitration
rearomatizaton
- E1 like process in which HSO₄ deprotonates the cation, transferring the bond to the carbocation
- completes the substitution and regenerates catalyst
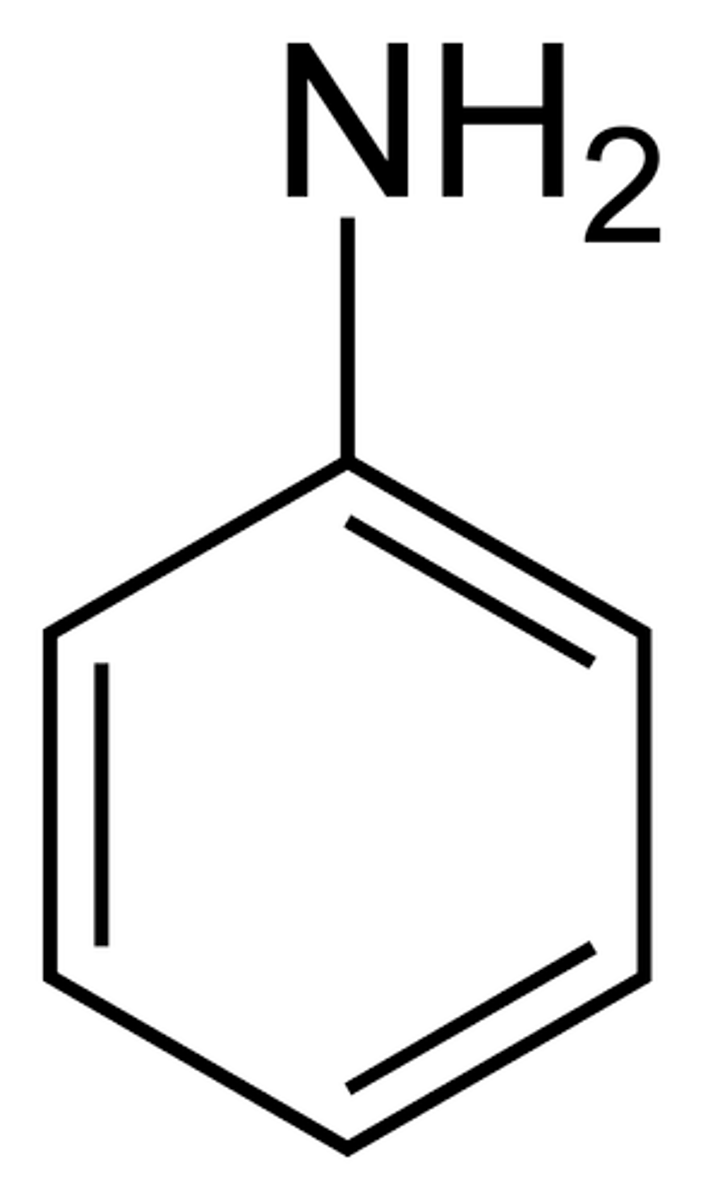
aniline
benzene with NH2
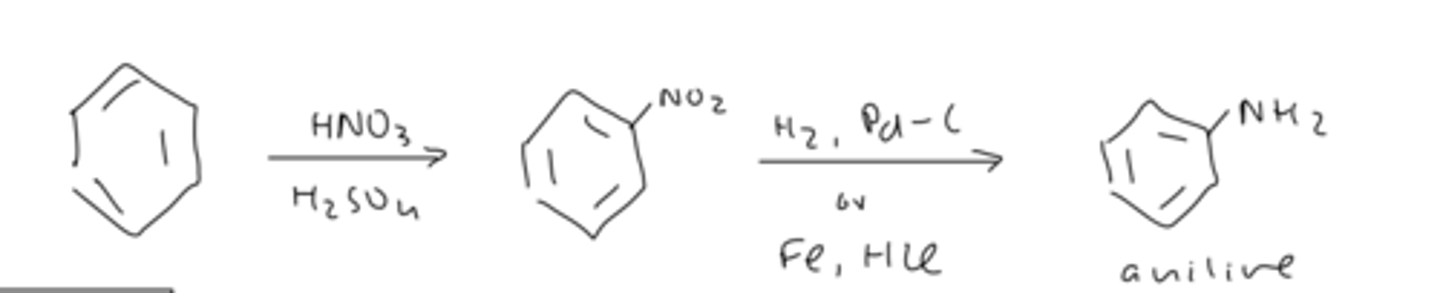
how to form aniline
react benzene with
1) HNO₃ and H₂SO₄
2) H₂, Pd-C or Fe, HCl
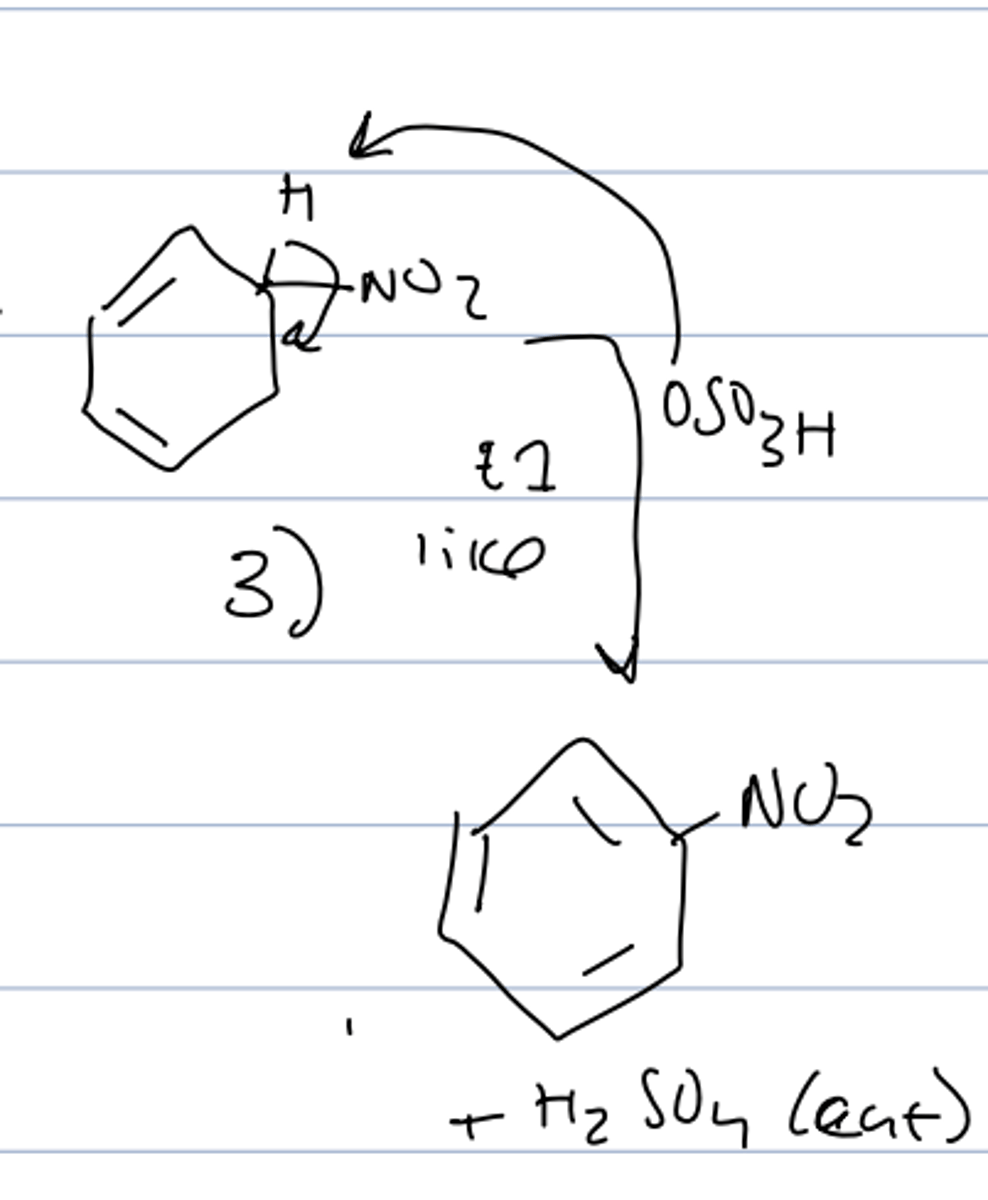
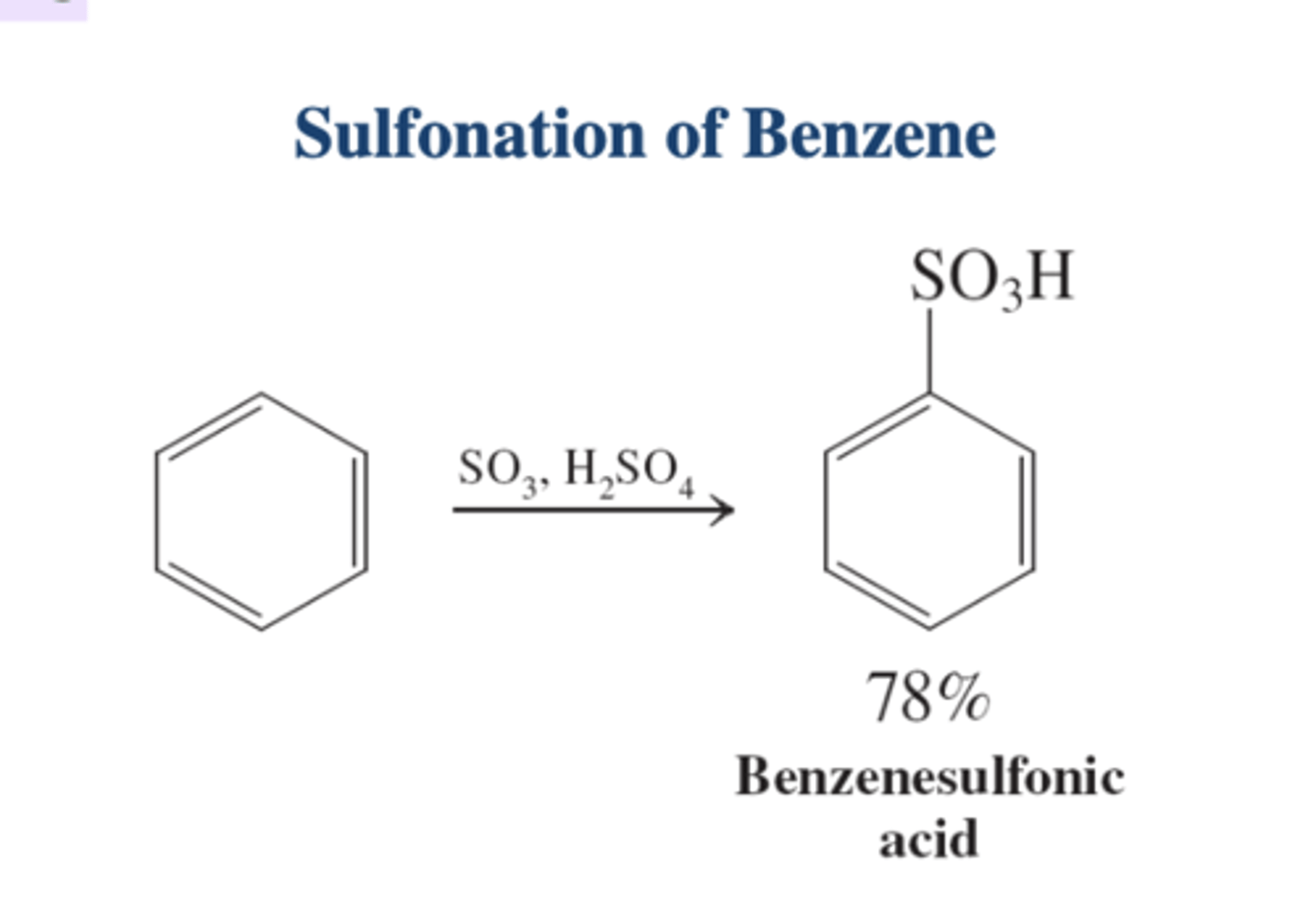
sulfonation overall reaction
benzene + H₂SO₄, SO₃ (8%)
step 1 of sulfonation
- no electrophile activation needed
- fuming sulfuric acid is an active electrophile
step 2 of sulfonation
- slow RDS
- pi bond attacks the SO₃
- forms a arenium ion with resonance
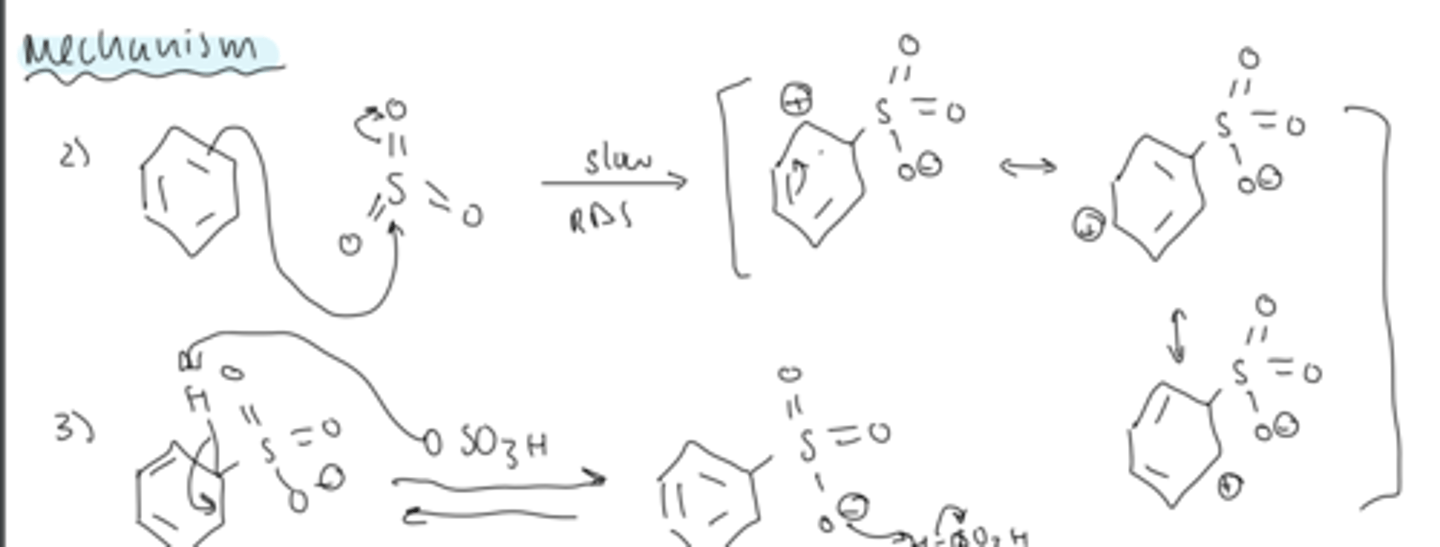
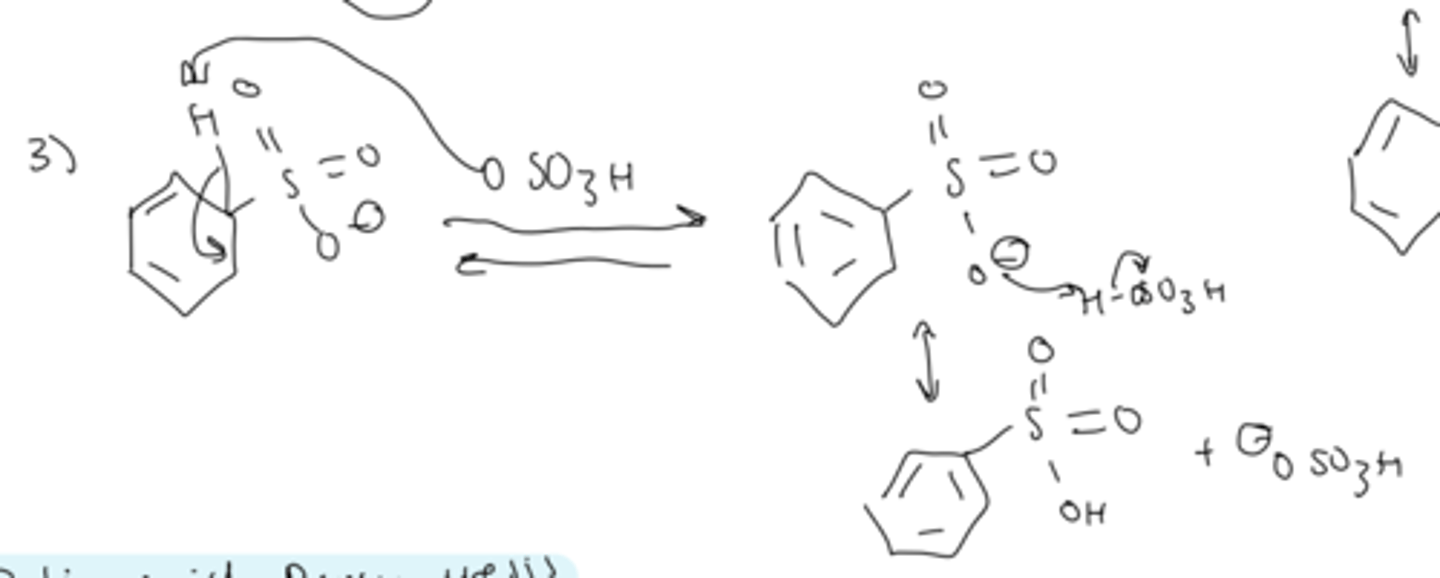
step 3 of sulfonation
- deprotonation by HSO4 regenerates the aromaticity
- protonation of one of the oxygens gives the final product
folic acid biosynthesis
sulfonation reversibility
reversible reaction: hydrolysis
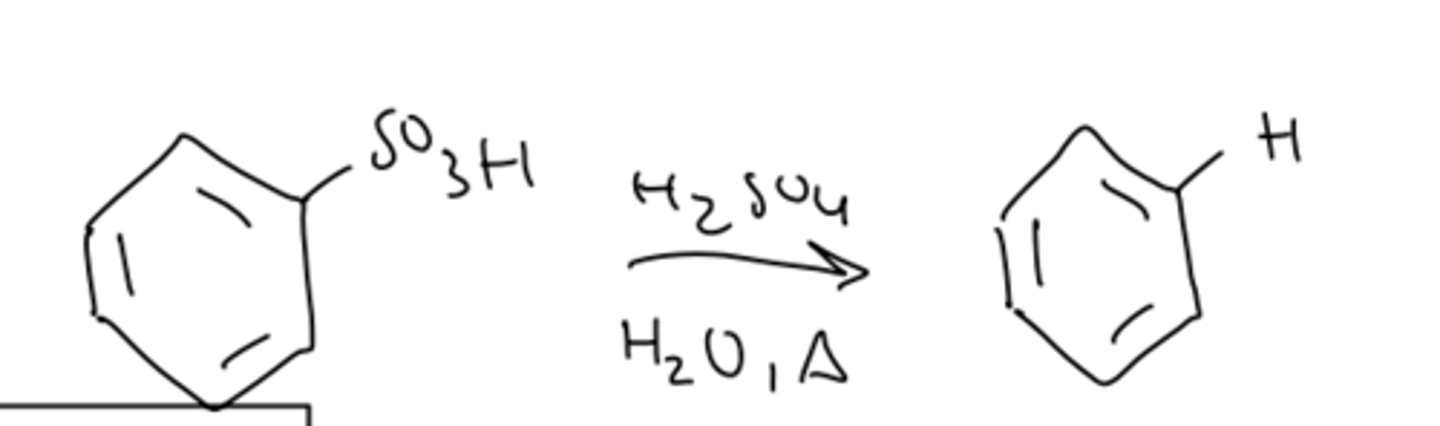
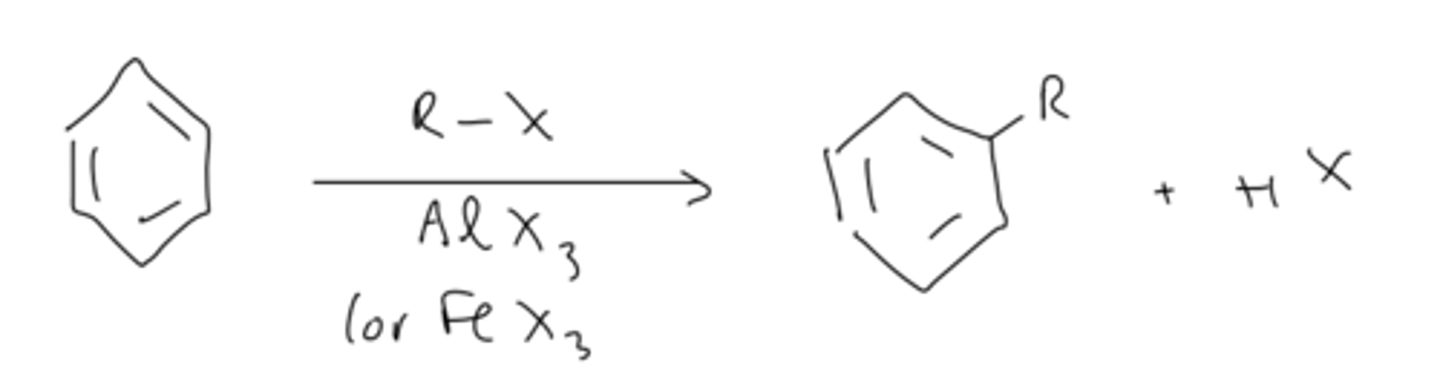
fridel-crafts alkylation overall reaction
benzene reacts with R-X and lewis acid catalyst (AlX3 or FeX3) to form a new carbon-carbon bond and H-X
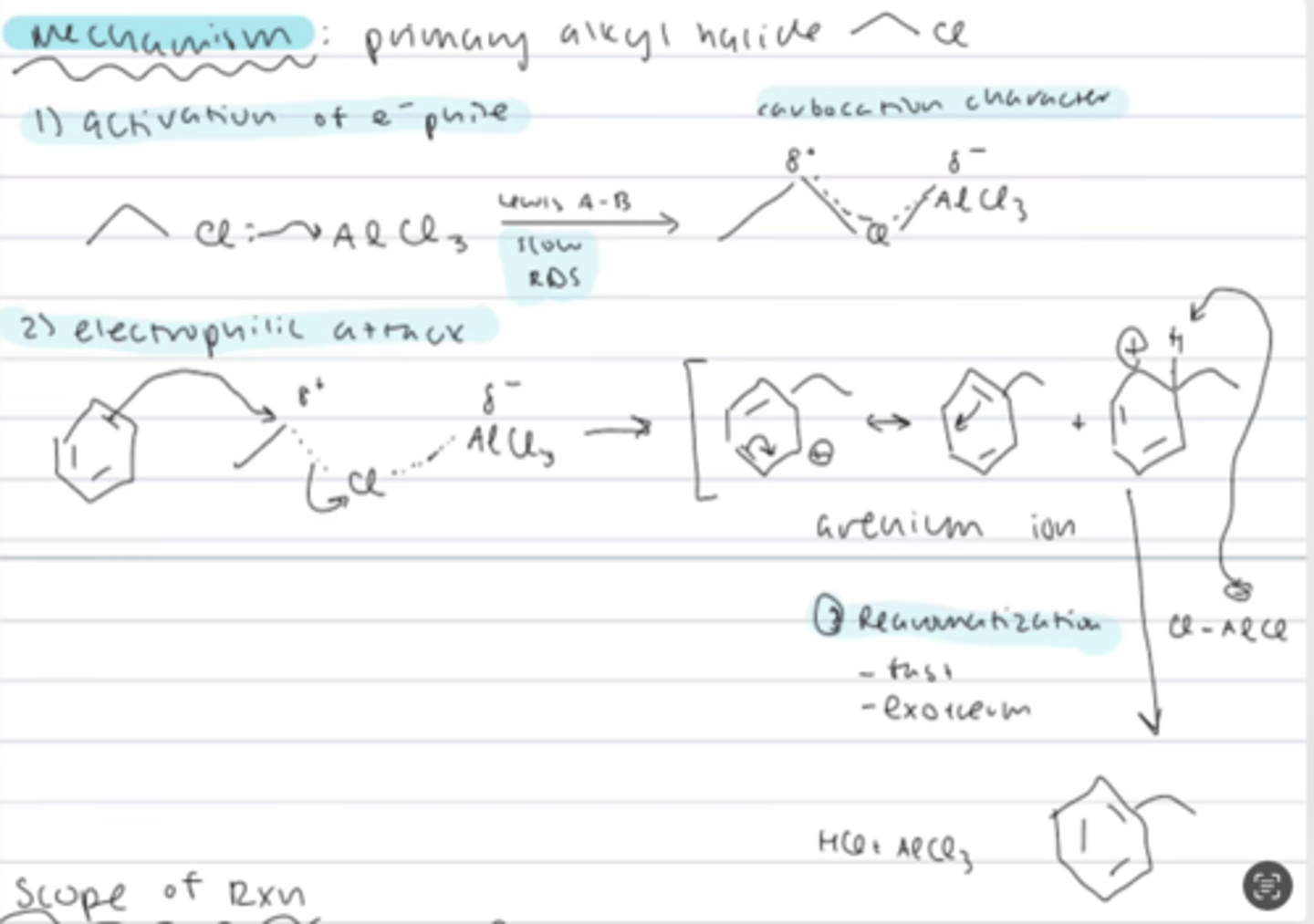
mechanism of friedel-crafts alkylation (primary haloalkane)
1) activation of the electrophile: halogen of the alkyl halide attacks the AlX3 = slow RDS (lewis A-B) to form an intermediate with carbocation character
2) electrophilic attack on the positively charge R, kicking out AlCl4, forming arenium ion
3) rearomatization (fast and exothermic) through deprotonation, regenerating the catalyst, forming HCl, and the new C-C bond to benzene
scope of friedel-crafts alkylation
1) intramolecular reaction
2) alcohols as precursor
3) alkenes as carbocation precursors
4) epoxide
friedel-crafts alkylation: intramolecular reaction
can be used to fuse a new ring onto the benzene
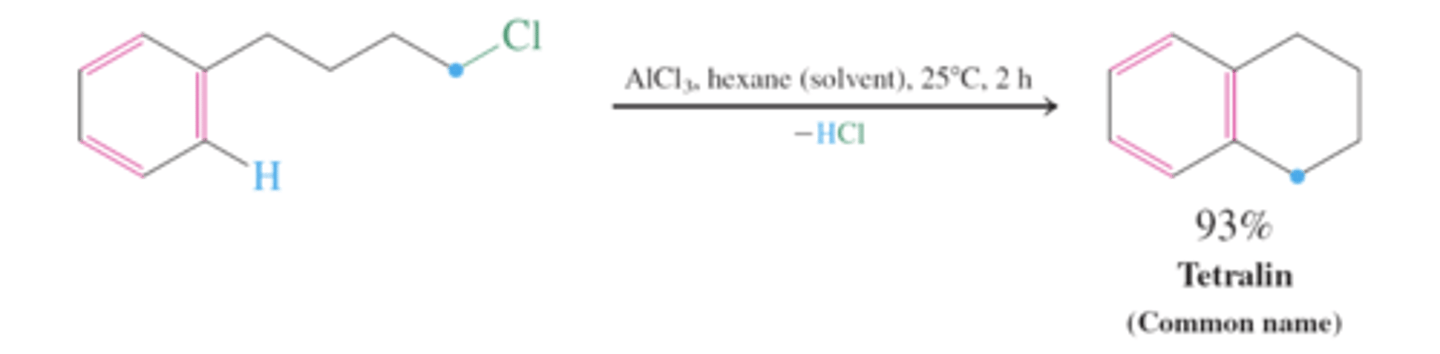
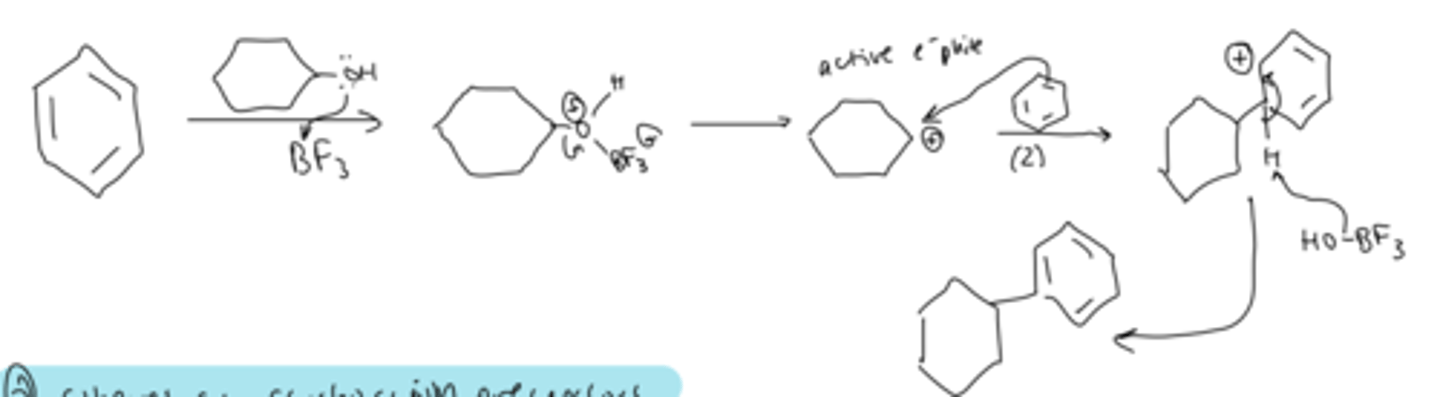
friedel-crafts alkylation: alcohols as (carbocation) precursor
treating alcohol with BF3 forms a carbocation which can be attacked by the benzene pi bond and deprotonated
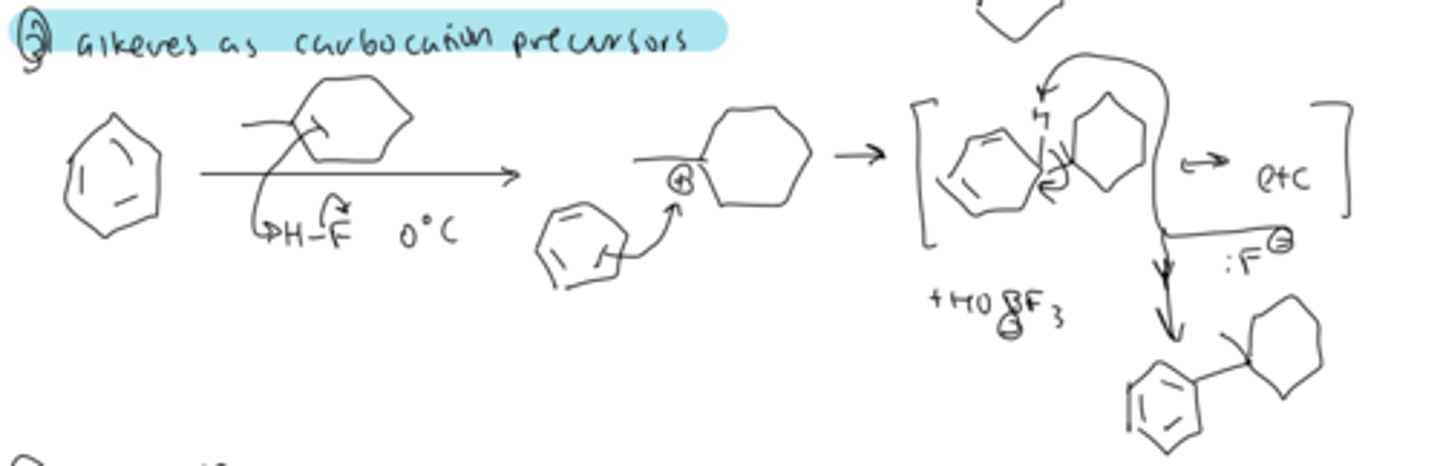
friedel-crafts alkylation: alkenes as precursors
treating alkene with H-X can form a carbocation that gets attacked by benzene and deprotonated by the X-
limitations of friedel-crafts alkylation
1) carbocation rearrangements
2) polyalkylation
3) FC rxns do not work on deactivated pi systems
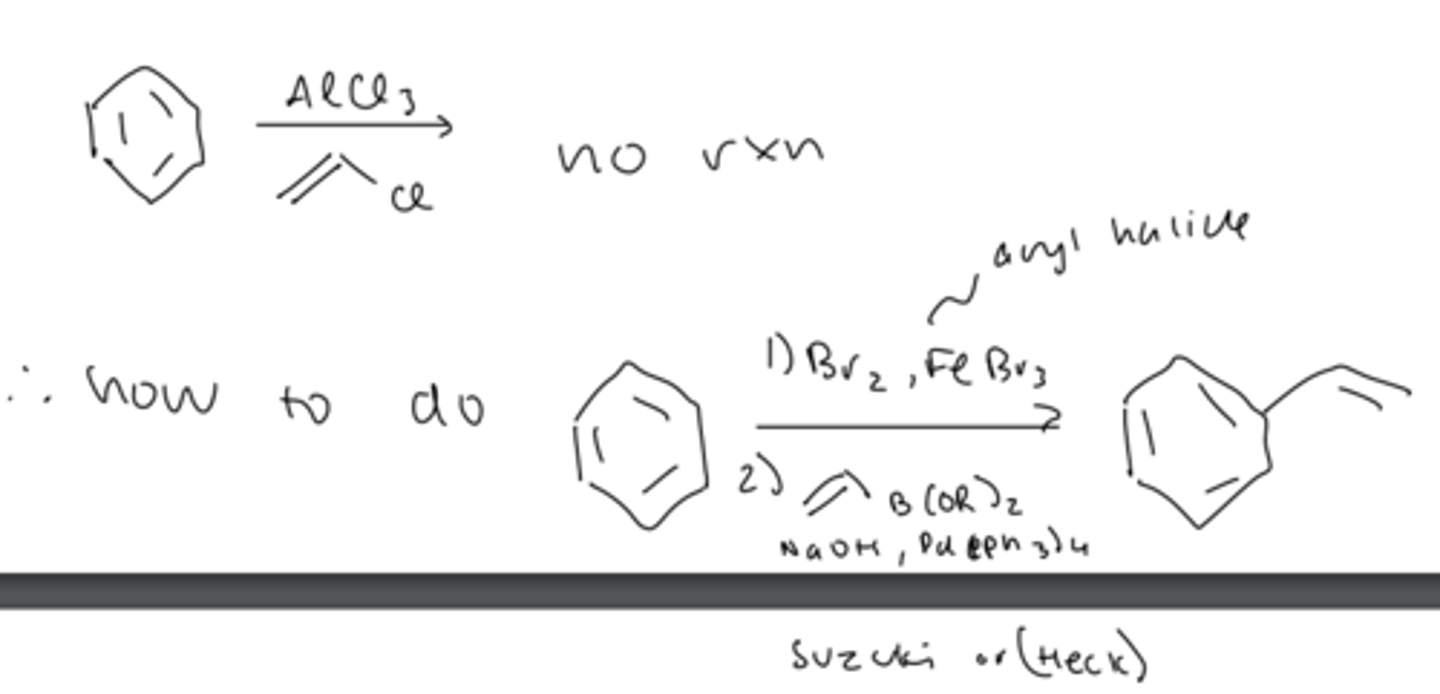
4) vinyl/aryl halides do not work
limitation #1 FC alkylation: carbocation rearrangement
the carbocation formed can undergo rearrangements (alkyl or hydride shifts) to form a more stable carbocation, changing the ratio of major and minor products
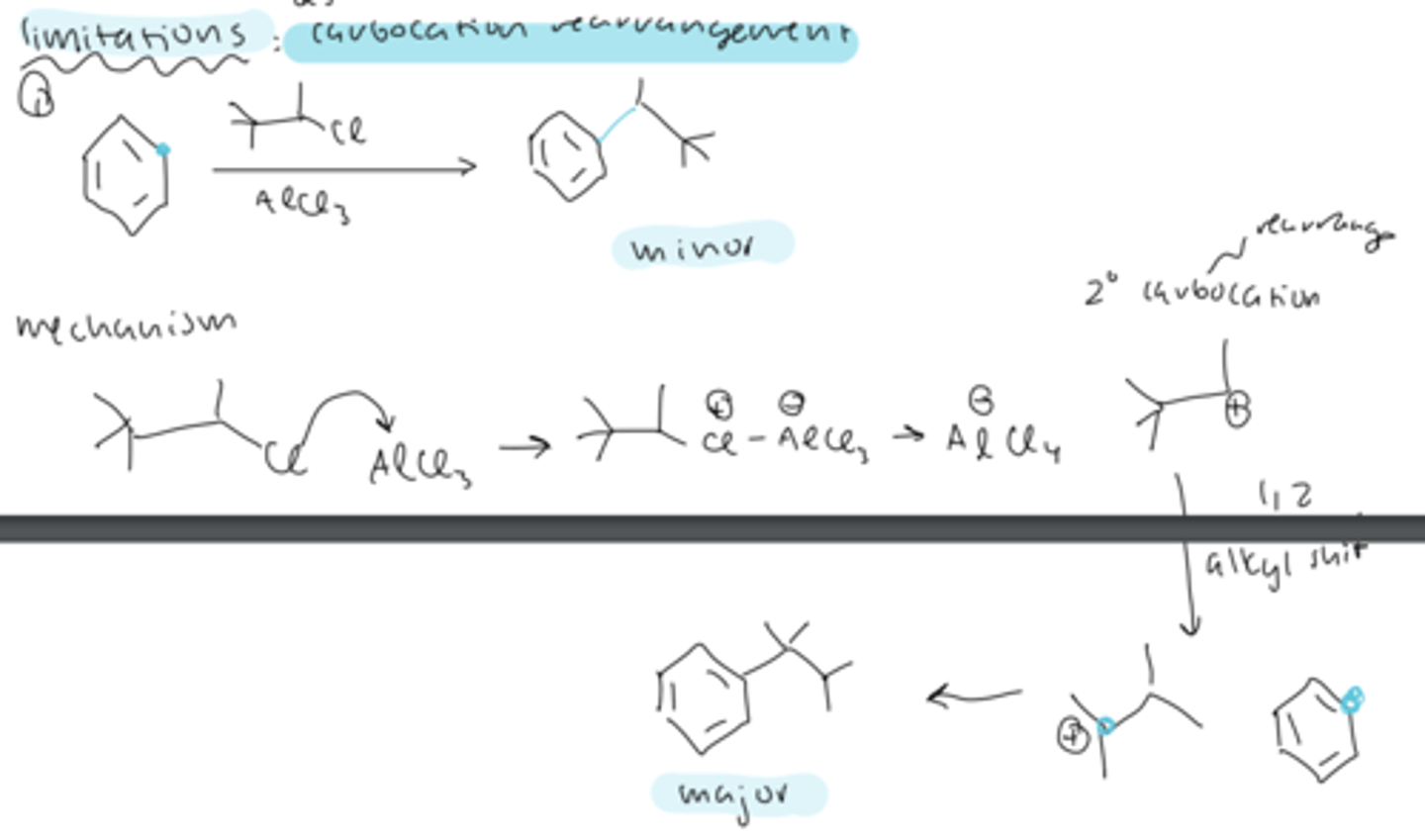
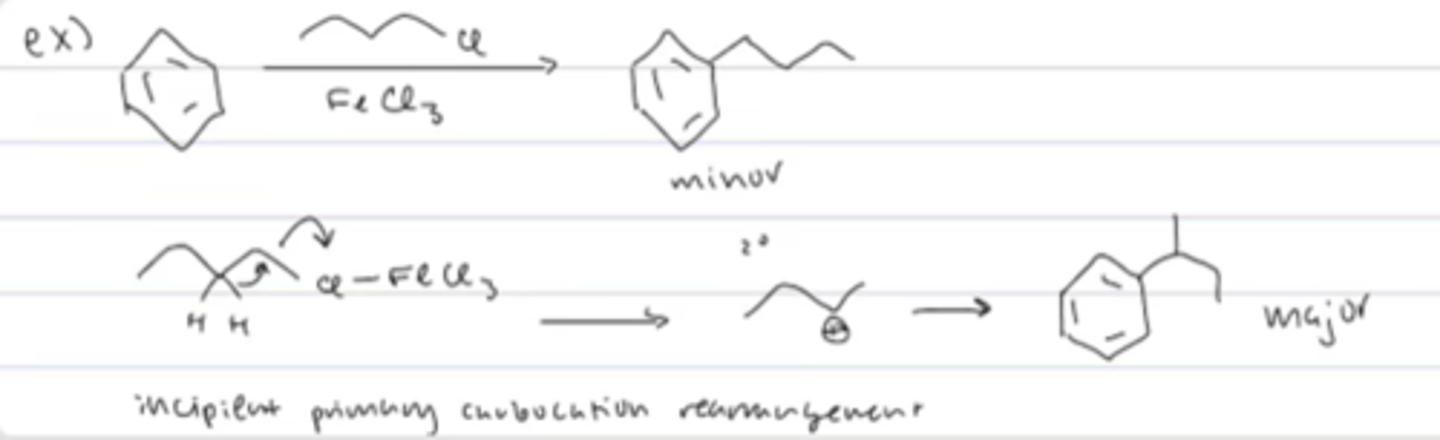
FC carbocation rearrangement with primary alkyl halide
incipient primary carbocation rearrangement: simultaneous hydride shift with the leaving group leaving
limitation #2 FC alkylation: polyalkylation
- benzene can be alkylated to form toluene
- toluene is more reactive than benzene because of the added alkyl substituents
limitation #3 FC alkylation does not work on deactivated systems
- electron withdrawing groups (EWGs) slow rxn
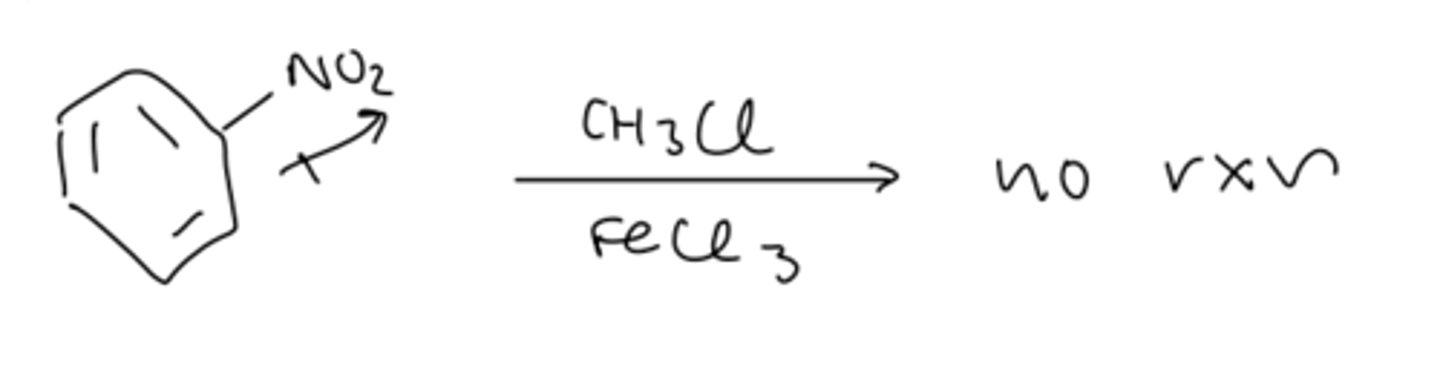
limitation #4 FC alkylation does not work with vinyl/aryl halides
- can be done by using a Suzuki (or Heck) reaction
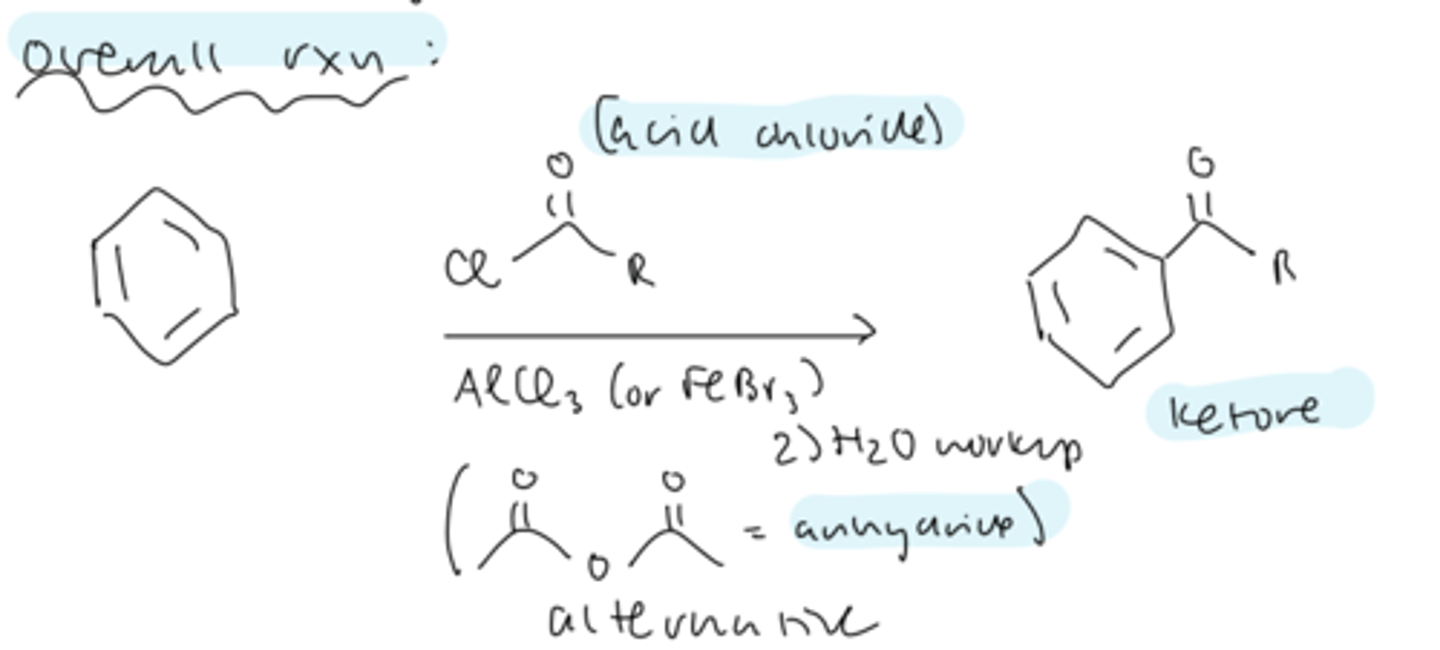
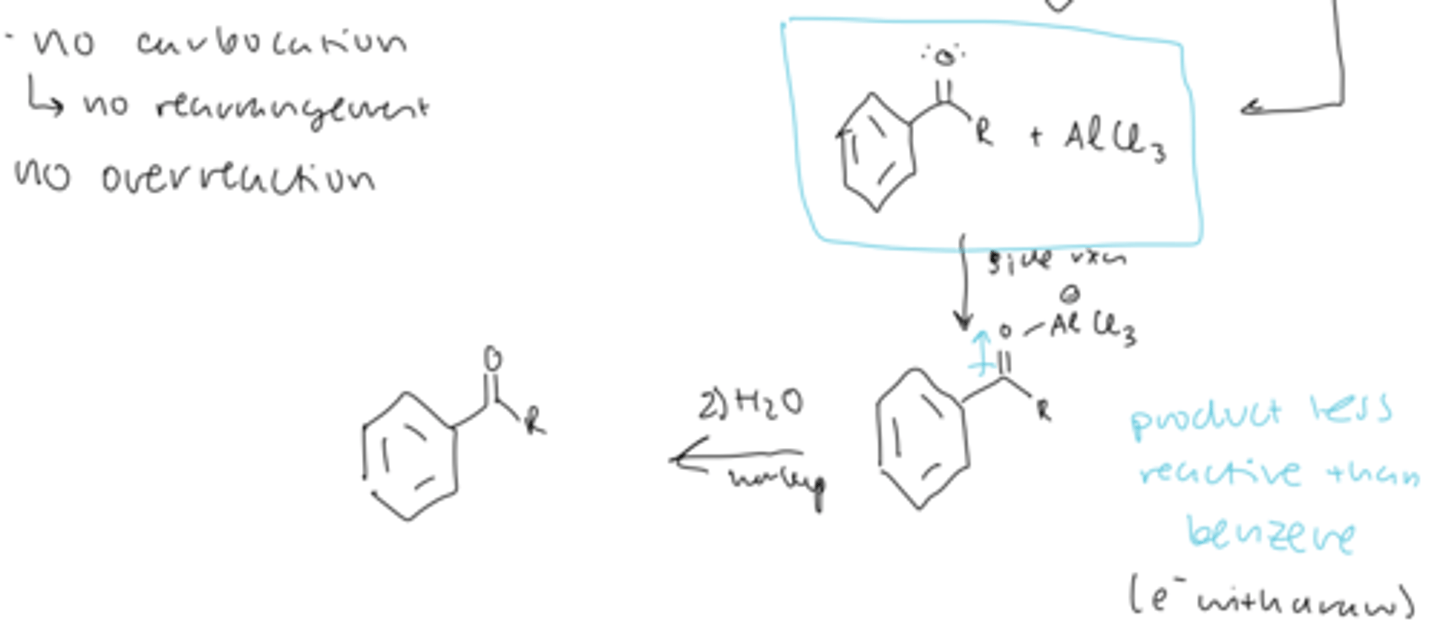
FC acylation is
more useful than alkylation
FC acylation overall reaction
benzene --> ketone
treat benzene with acid chloride, AlCl3 or FeBr3
then perform aqueous workup
mechanism of FC acylation
1) activation of electrophile: acid chloride loses a Cl to AlCl3 to form an acylium ion and AlCl4
2) electrophilic addition: benzene can attack the acylium ion to form a carbocation
3) deprotonation by Cl of AlCl4 forms the ketone and AlCl3
4) aqueous workup after side reaction
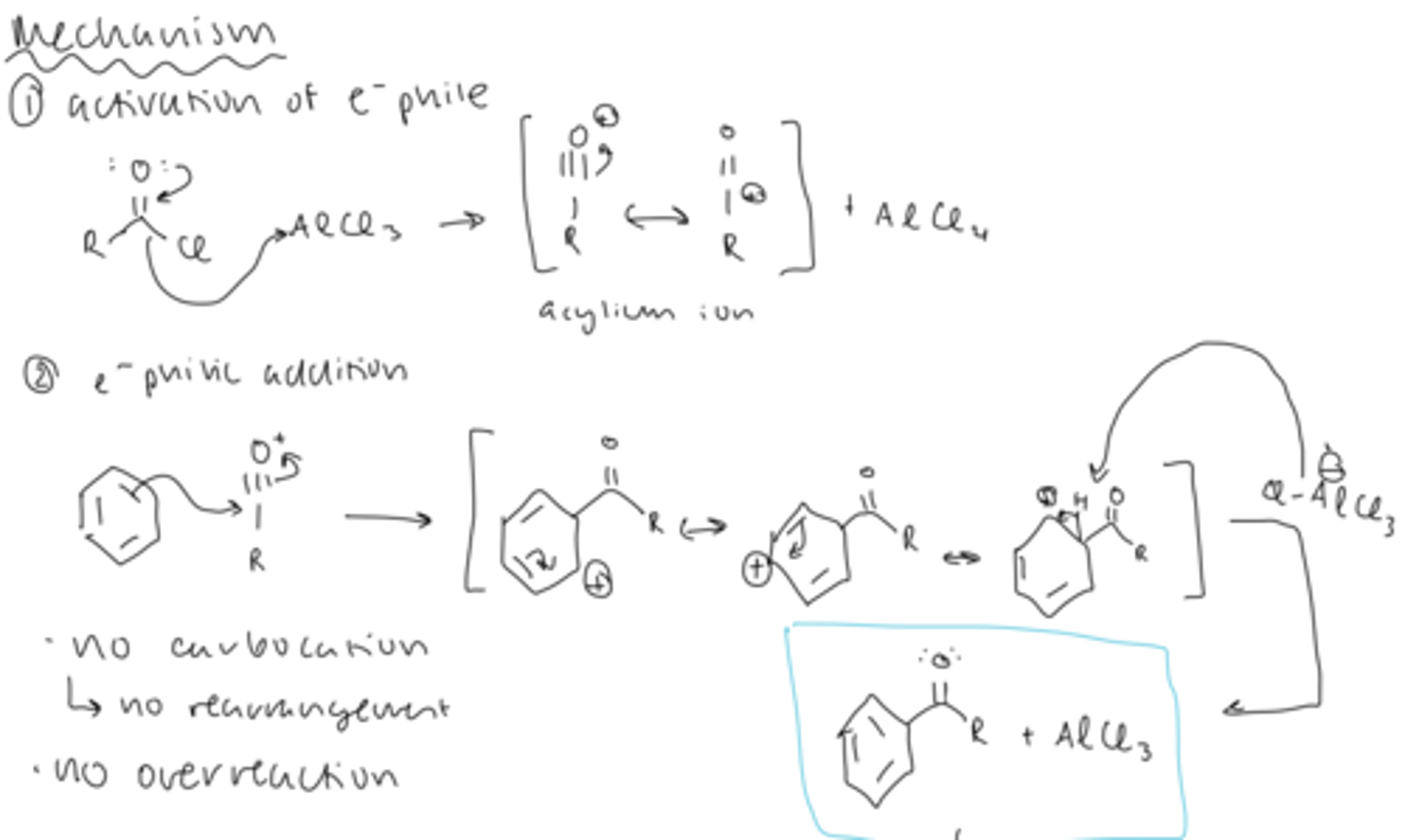
side reaction of FC acylation product
- the ketone and AlCl3 can react to produce a product less reaction that benzene (EWG)
- so aqueous workup is necessary to reform the ketone
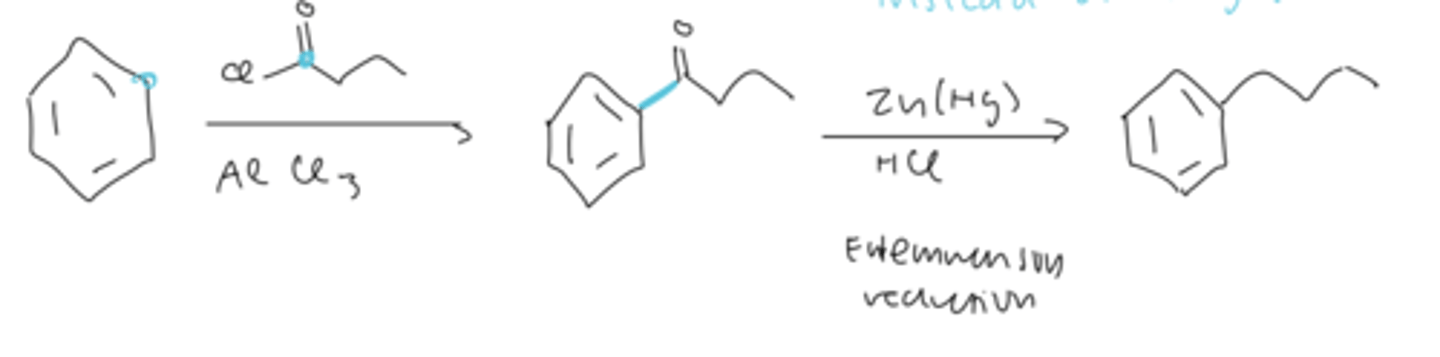
clemmenson reduction
an alternative to alkylation using FC acylation
- after acylation to form ketone, treat with Zn(Hg) and HCl to form the alkane substituent on benzene