Immune Response Against Tumors and Transplants
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
T cells
CD 4 T helper cells-cytokines: mediate DTH reaction
CD8 T cells: mediate cytotoxic functions (killers)
Recall cross-presentation of antigens
MHC molecules
Antibodies
Mediate ADCC via NK cells
Mediate complement activation - lysis and inflammation
Tumors
Sloppy immune response
Transplants
Immune system reacts
Common features of tumors and transplants
Immune system responds to noninfectious cells and they are recognized as foreign
CTL’s destroy tumor cells and transplants
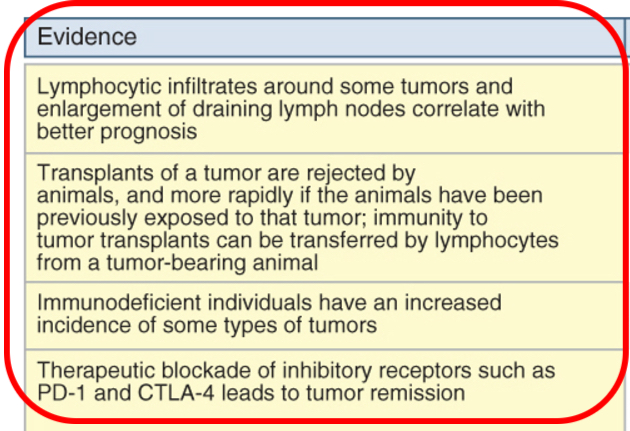
Immune surveillance
Adaptive immune system can prevent the outgrowth of transformed or tumor cells. Yet, tumors can develop in healthy individuals.
Immune response against tumors
Tumor antigens
Malignant tumors express various types of molecules that may be recognized by the immune system as foreign
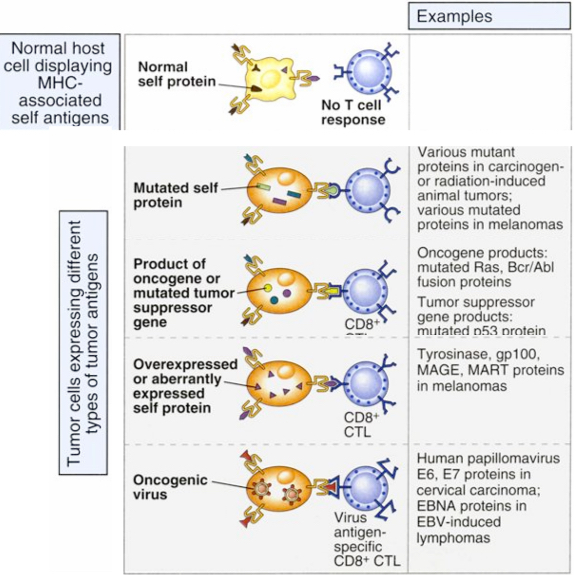
Types of tumor antigens recognized by T cells
Tumor antigens that are recognized by tumor-specific CD8+ T cells may be mutated forms of normal self proteins, products of oncogenes or tumor suppressor genes, overexpressed or aberrantly expressed self proteins, or products of oncogenic viruses.
Tumor antigens may also be recognized by CD4+ T cells, but less is known about the role that CD4+ T cells play in tumor immunity.
Cells involved in tumor cell killing
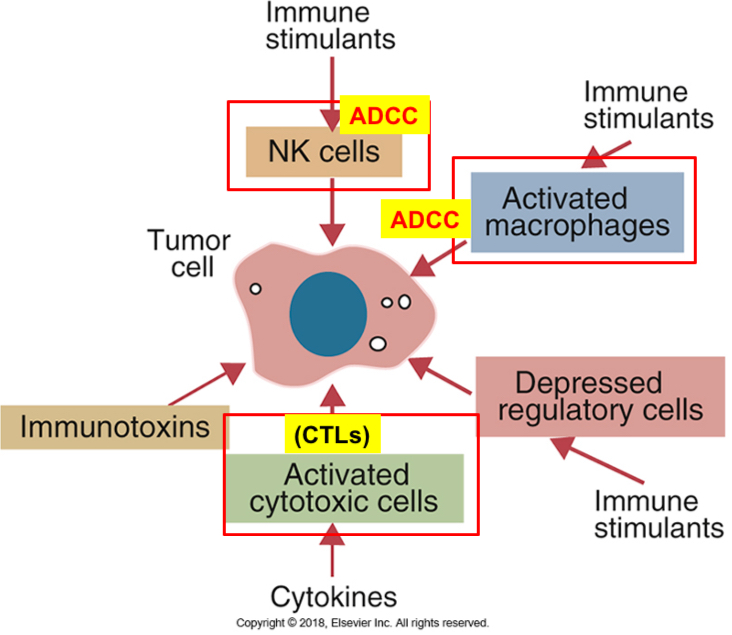
Immune mechanisms to tumor rejection or destruction
The principal mechanisms involves killing of tumor cells by CTL’s specific to tumor antigens. Majority of tumor antigens in tumor patients are endogenously derived cytosolic proteins; displayed by the MHC class l
NK cells in tumor immunity: ADCC
Macrophages: produce inflammatory cytokines (Ex: TNF-alpha, NO, phagocytosis, and ADCC)
Cross presentation
CTL responses are induced by recognition of tumor antigens on host antigen presenting cells
CD8+ T cell responses to tumors may be induced by cross-priming (also called cross-presentation), in which the tumor cells or tumor antigens (or both) are taken up by dendritic cells, processed, and presented to T cells.
In some cases, B7 costimulators expressed by these antigen-presenting cells (APCs) provide the second signals for the differentiation of the CD8+ T cells.
The APCs may also stimulate CD4+ helper T cells, which may provide signals for CTL development.
Differentiated CTLs kill tumor cells without a requirement for costimulation or T cell help. CTL, Cytotoxic T lymphocyte
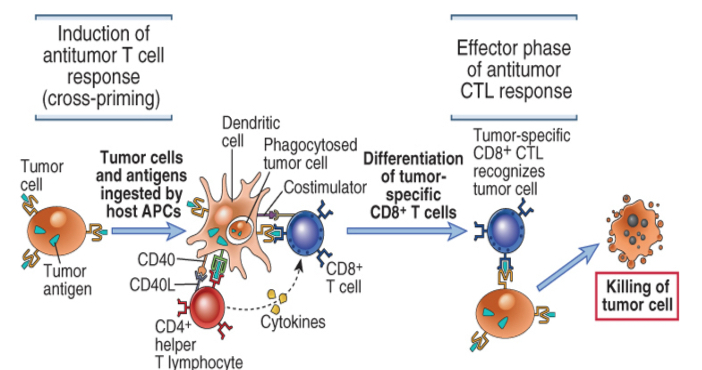
Cytotoxic T lymphocyte responses against tumors
Tumor antigens are picked up by host dendritic cells, and responses are initiated in secondary lymphoid organs. Tumor-specific cytotoxic T lymphocytes migrate back to the tumor and kill tumor cells. CD4+ T cell responses against tumors involve similar initiating steps to generate tumor-specific helper T cells, but the antitumor effector mechanisms are different.
NK cells markers
CD56 is one of the major markers of NK cells
Bovines: CD2+, CD5+, WC1+, MHC class ll+, and asialo-GM1+
Pigs: CD2+, CD8+, MHC class ll+, LFA-1+
ADCC by macrophages and NK cells
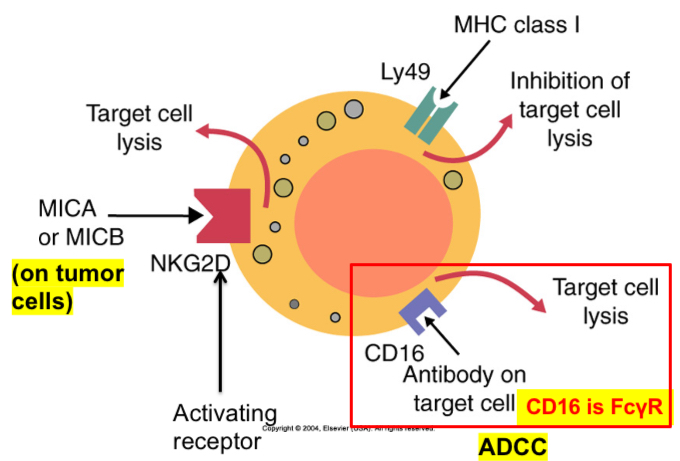
Three of the receptors found on mouse NK cells. Ly49 recognizes MHC class I molecules and suppresses NK cytotoxicity. CD16 binds immunoglobulins and triggers cytotoxicity by antibody-dependent cellular cytotoxicity. NK cells also express NKG2D, a receptor for molecules such as MICA and MICB. These molecules are commonly expressed on tumor cells.
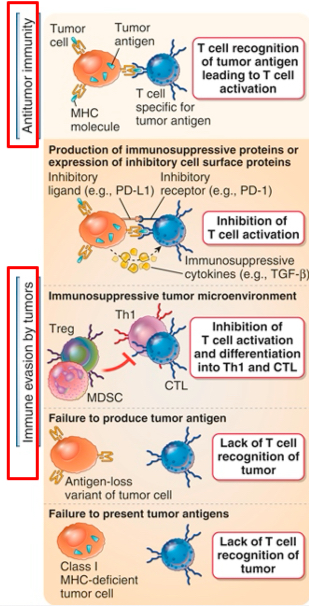
Evasion of immune responses to tumors
Tumors can evade immune attack
Immune response may be weak because most tumor antigens are poor immunogens and they differ only slightly from self-antigens
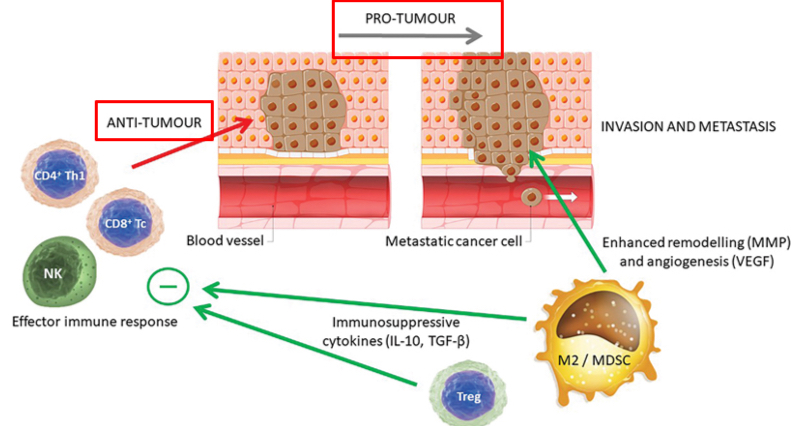
Immune response to tumors
In addition to appropriate cytotoxic effector immune responses to tumors, there are counteractive responses that may enhance the malignant potential of cancer. These include the presence of Treg cells within the population of tumor-infiltrating lymphocytes and the presence of TAMs of the M2 phenotype that may encourage the growth, invasion, and metastasis of the tumor.
Outcomes to response to tumors
Tumors stop expressing the antigens (antigen-loss variants)
Tumors stop expressing MHC class l molecules; peptides are not displayed for CTL’s
Immune suppression:
Cytokines (TGF-beta)
Express inhibitory receptors (CTLA-4 and PD-1)
Treg cells/myeloid derived suppressor cells (MDSCs)
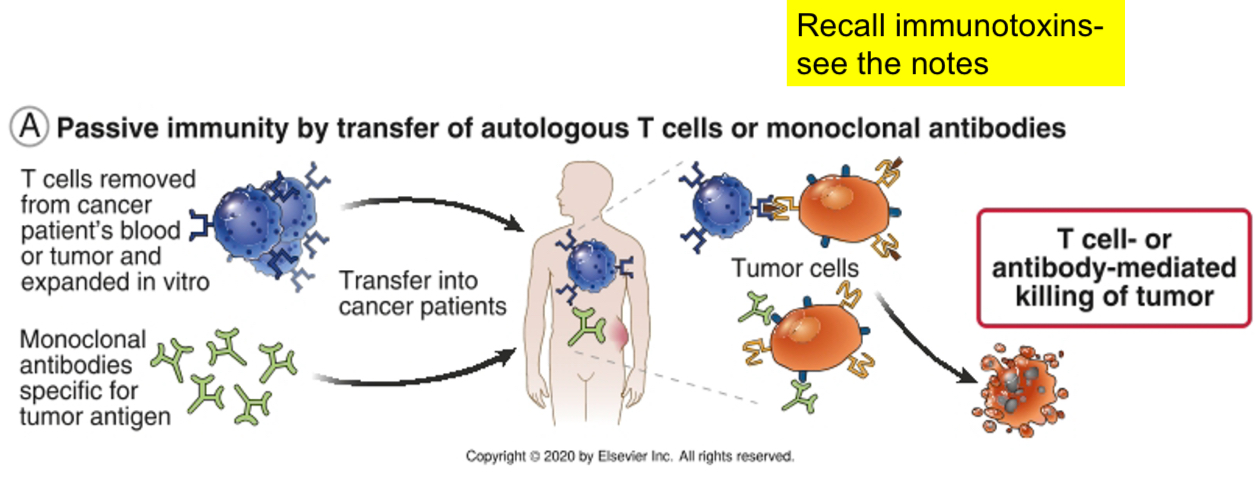
Strategies for enhancing antitumor immune responses
Injecting monoclonal antibodies to tumor antigens that are coupled with toxins: antibodies activate phagocytes or the complement system or deliver the toxins to the tumor cells (immunotoxins-magic bullets or guided missiles).
Bacterial toxins commonly used in immunotoxins include Diphtheria toxin (DT) and the toxin from Pseudomonas exotoxin (PE). Plant toxins utilized in immunotoxins include the A chain of ricin (RTA), and the ribosome inactivating proteins (RIPs) gelonin, pokeweed antiviral protein, and dodecandron.
Schematic representation of adoptive therapy
Lymphokine-activated killer (LAK) - mostly NK cells: Produced by incubating blood lymphocytes in the presence of IL-2 for 4 to 7 days.
Tumor-infiltrating lymphocytes (TILs): CTLs and NK cells
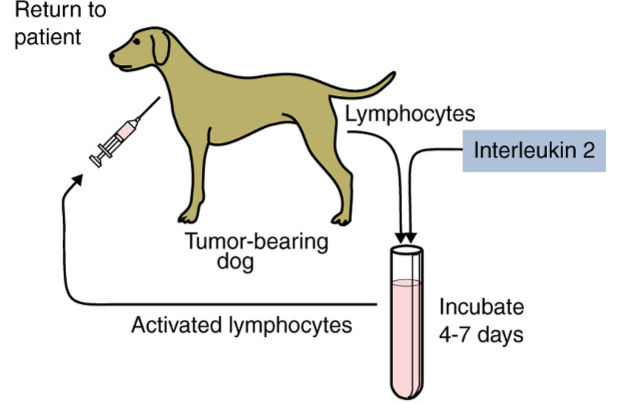
Strategies for enhancing antitumor immune responses
Dendritic cells vaccines:
Dendritic cells (DCs), generated in vitro from blood monocytes taken from a patient with a tumor can be pulsed with defined tumor antigens and infused back into the patient, where they will present the antigen to T cells specific for that antigen and boost a tumor-specific immune response. In other approaches, the DCs are transfected with a gene encoding the tumor antigen and sometimes also a cytokine that promotes immune responses, and these cells are used as vaccines.
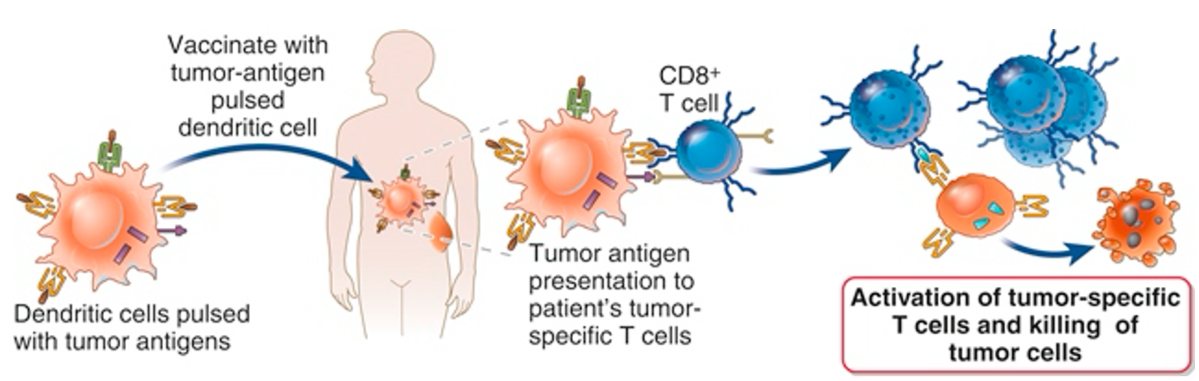
Overview of predictive biomarkers for response to ICIs
The response to immune checkpoint inhibitors varies depending on the TME. In the responders, tumors have a high neoantigen load, high levels of TILs, especially effector cells, a high Teff to Treg ratio, low MDSC levels and increased secretion of IFN-γ and other cytokines.
In nonresponders, the TME contains high levels of immunosuppressive cells, such as Tregs and MDSCs, and very low levels of NK cells and activated lymphocytes
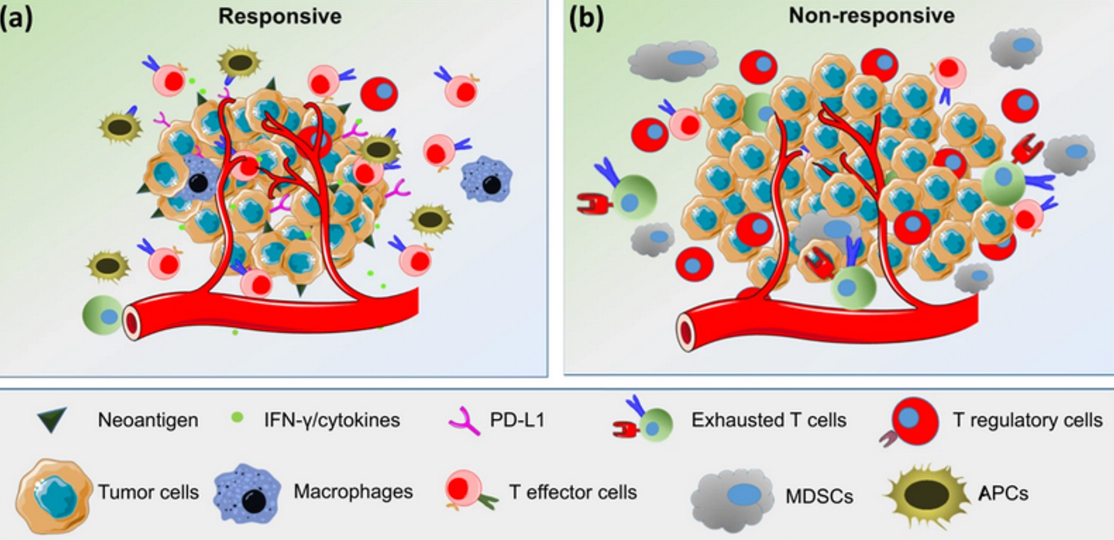
Tumor immunotherapy by immune checkpoint blockade
In lymphoid tissues, inhibition of cell-mediated immunity (CMI) can occur when tumour antigens are presented in the context of inhibitory (CTLA-4), rather than co-stimulatory (CD28), signals. Administration of anti-CTLA-4 antibody can inhibit this effect and enhance activation of tumour-specific naïve CD8+ T cells. Within the tumour microenvironment, expression of inhibitory molecules on the tumour cell surface (PD-L1, PD-L2) can inhibit the activity of CD8+ cytotoxic T lymphocytes (CTLs). Use of monoclonal antibodies directed against either PD-L1 or alternatively PD-1, expressed on the T cell, can abrogate this effect and permit their normal function of cytotoxicity.
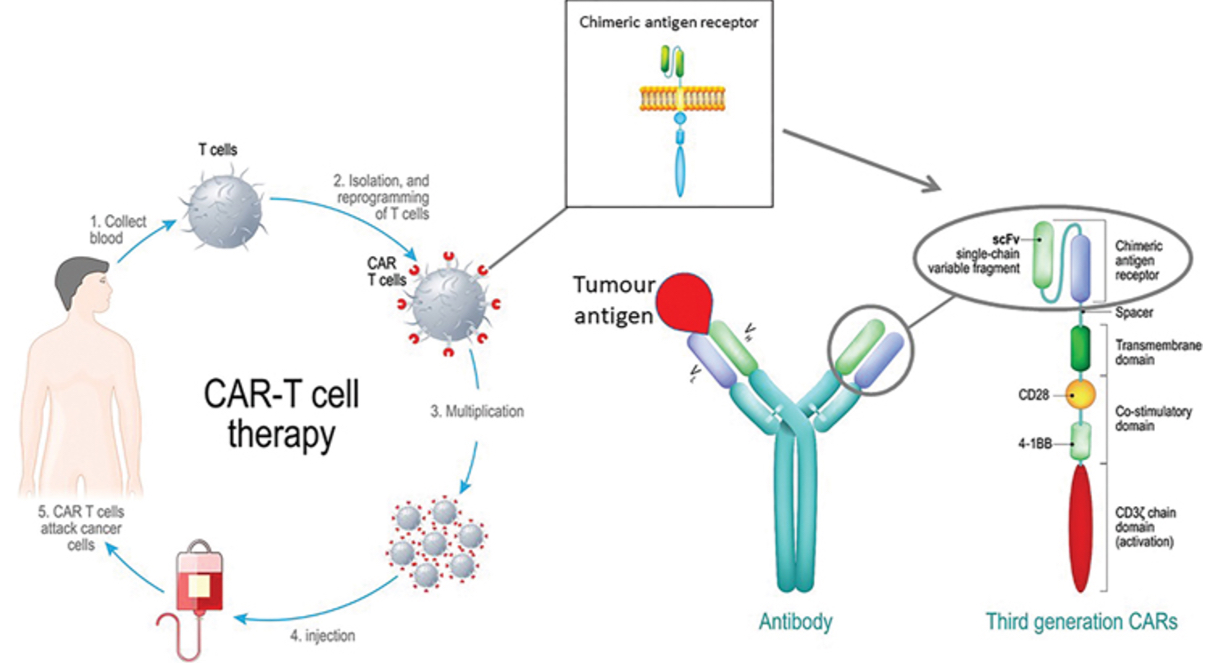
Chimeric Antigen Receptor (CAR) T cell therapy
T cells can be isolated from the patient’s blood and engineered to express a recombinant chimeric antigen receptor containing a single-chain Fv, derived from a monoclonal antibody that recognizes a tumour antigen target.
This is combined with a transmembrane domain to anchor the molecule to the T cell surface and intracellular signalling domains that mimic signal 1 and signal 2 that would normally be delivered via the TCR/CD3 complex and CD28.
The ‘reprogrammed’ CAR T cells are expanded in vitro then infused back into the patient, where they may target and destroy cancer cells.
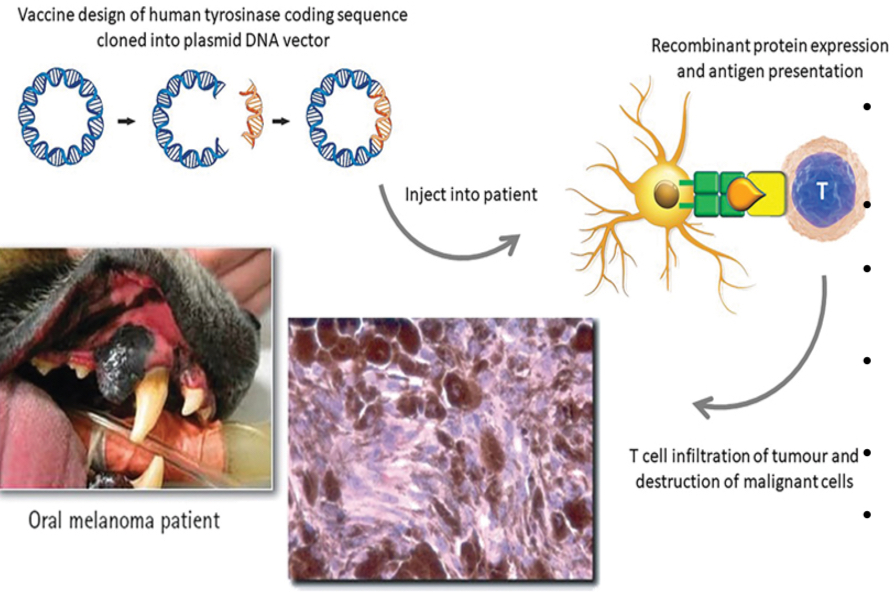
DNA vaccine against canine melanoma
Canine melanoma is a tumor that is very aggressive and poorly responsive to chemotherapy
It is a xenogenic DNA vaccine = DNA for human tyrosinase injected into dogs
Tyrosinase, an overexpressed protein of the melanoma
Immune system is tolerant to canine tyrosinase but not human tyrosinase
Inserted human DNA for tyrosinase into a bacterial plasmid
Vaccinate dogs with the xenogenic DNA plasmid
Activates antibody production and CTL response (can recognize canine tyrosinase)
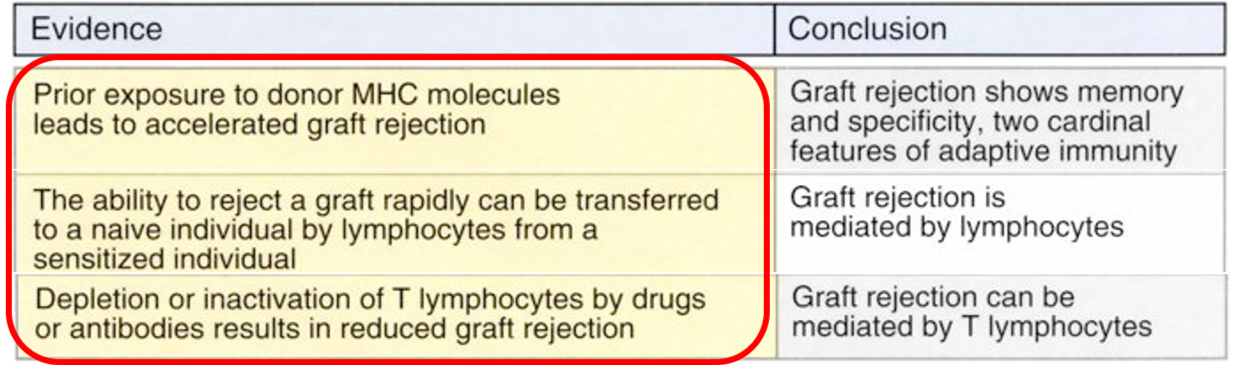
Immune responses against transplants
Donor
Individual who provides (donates) the graft
Recipient (host)
Individual in whom the graft is placed
Syngeneic
Grafts exchanged between two inbred mice of the same strain bearing the identical MHC molecules. It is analogous to transplantation between two genetically identical monozygotic twins (isograft)
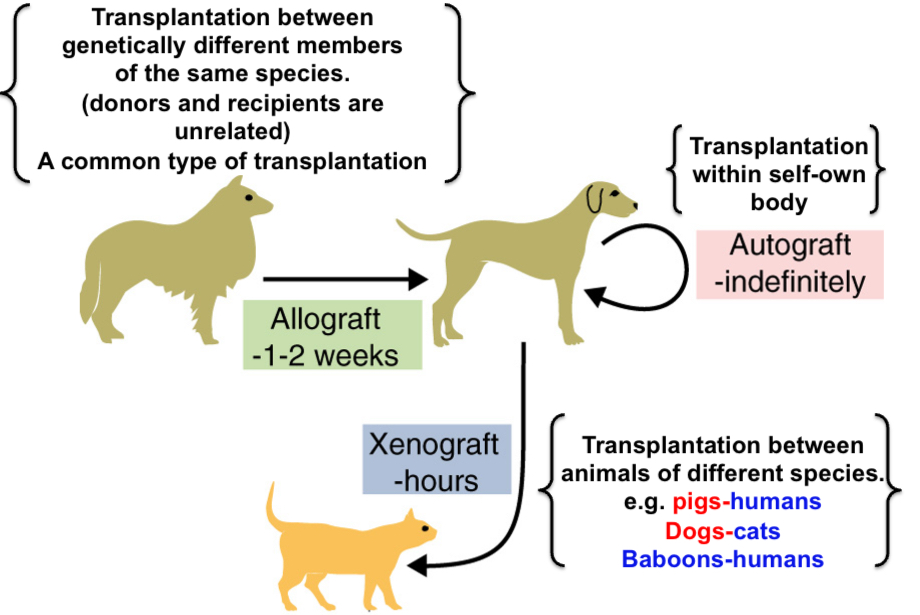
Autograft
Self tissue transferred from one body site to another in the same individual (within self)
Allogenic animals or allograft
Animals (and grafts) of one species that differ from other animals of the same species (Ex: grafts between breeds within the species - dogs, cats, humans [within species])
Xenogeneic animals or xenografts
Animals (and grafts) of one species that differ from other animals of different species (Ex: pig-human) (between species)
Allografts are always
rejected
Antigens that serve as the targets of rejection are called
alloantigens and xenoantigens
Antibodies and T cells that react against alloantigens and xenoantigens are called
alloreactive and xenoreactive antibodies and T cells, respectively
Differences among autografts, allografts, and xenografts
Transplantation antigens
Antigens of allografts that serve as the targets of rejection are proteins encoded in the MHC.
MHC genes are highly polymorphic: HLA-A genes - 120 and 250 alleles of HLA-B genes (estimation).
Every individual is likely to express some MHC proteins that appear foreign to another individual’s immune system except identical twins.
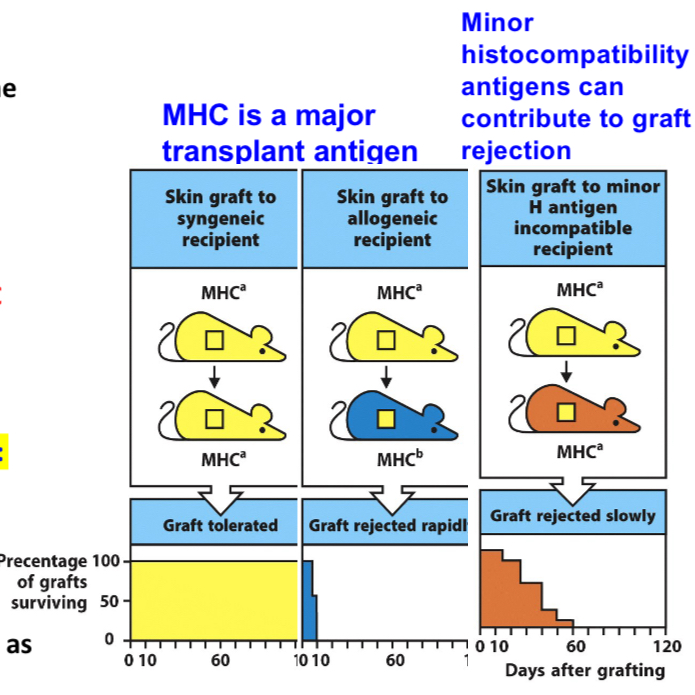
MHC molecules are major targets of rejection
MHC molecules are recognized as foreign
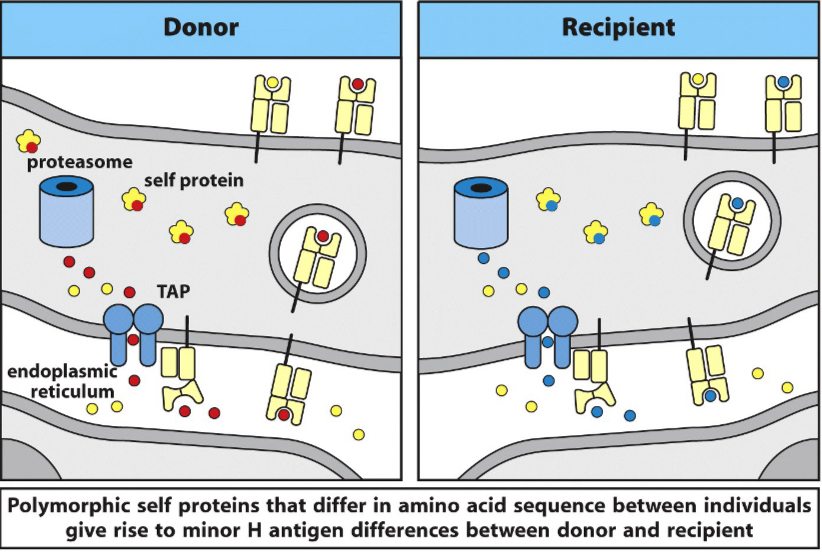
Non-MHC antigens can also induce graft rejection
Called minor histocompatibility antigens
Ex: allelic forms of normal cellular proteins
Their rejection reactions are not as strong as MHC proteins
Recognition of allogeneic MHC molecules by T lymphocytes
Recognition of allogeneic MHC molecules may be thought of as a cross-reaction in which a T cell specific for a self MHC molecule–foreign peptide complex also recognizes an allogeneic MHC molecule whose structure resembles that of a self MHC molecule–foreign peptide complex. Peptides derived from the graft (labeled self peptide) may not contribute to allorecognition, or they may form part of the complex that the T cell recognizes. The type of T cell recognition depicted in B andC is direct allorecognition.
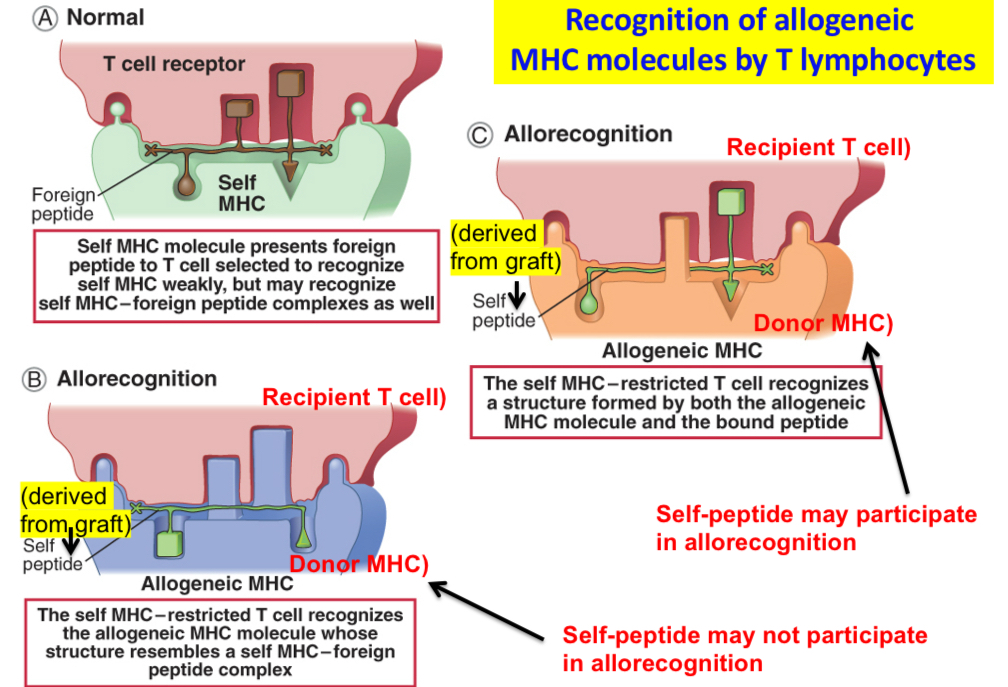
Transplantation antigens
•Self MHC restriction is the basis of T cell selection in the thymus: mature T cells are selected to recognize only peptides displayed by self MHC molecules.
•Mature T cells in the periphery strongly recognize self MHC molecules displaying foreign peptides.
•Allogeneic MHC molecules containing peptides derived from the allogeneic cells look like self MHC molecules + bound foreign peptides. Recognition of allogeneic MHC molecules in allografts is an example of immunologic cross-reaction.
•Non-MHC antigens can also induce graft rejection and they are called ‘minor histocompatibility antigens’ e.g. allelic forms of normal cellular proteins and their rejection reactions are not as strong as MHC proteins.
•Important targets in blood transfusion and bone marrow transplantation.
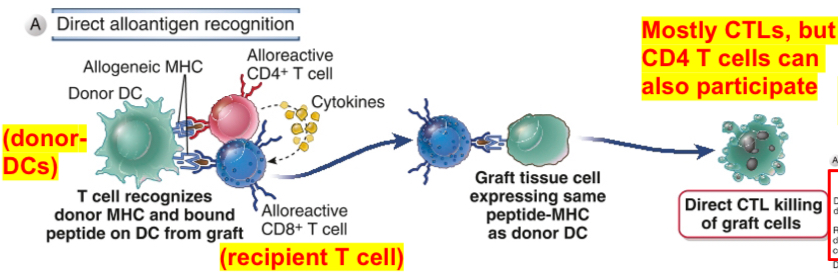
Direct allorecognition or direct presentation of alloantigens
Cells may recognize allogeneic MHC molecules in the graft displayed by professional APC derived from allogeneic grafts (donor) (donor’s MHC molecules are recognized directly by recipient’s T cells). This can happen only if the donor graft contains APC e.g DC.
Direct allorecognition stimulates the development of alloreactive T cells (mostly CTL) that recognize the cells of the donor graft. It is important for CTL-mediated acute rejection.
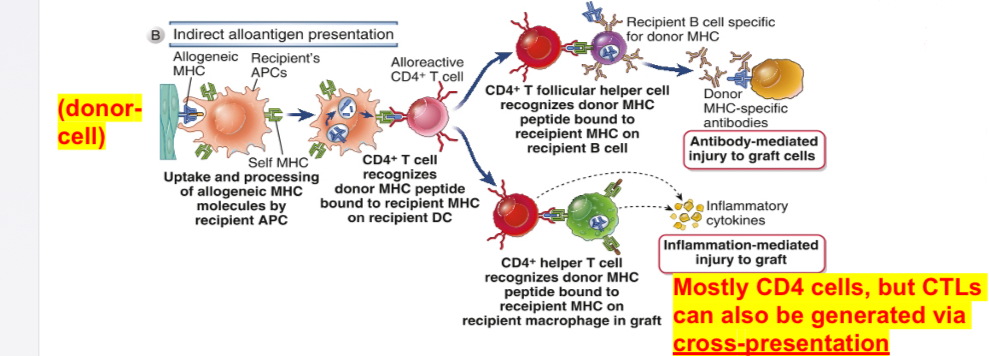
Indirect allorecognition (or indirect presentation)
Graft alloantigens (donor’s) are processed and presented by host’s (recipient) professional APC; mediators are mostly CD4 T helper cells causing DTH reaction; CD8 T cells (CTL) may also contribute to indirect allorecognition.
MHC molecules of donors are processed and presented by host’s (recipient) self MHC molecules; CD4 T cells respond to alloantigens acquired through the endosomal vesicular pathway as a consequence of phagocytosis and some antigens do enter MHC I pathway leading to the generation of CD8 T cells (an example of cross-presentation or cross priming).
Important in chronic rejection and also in the induction of T cell-dependent antibody responses to allogeneic proteins.
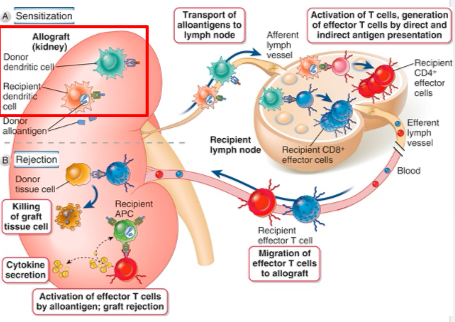
Activation of alloreactive T cells
In the case of direct allorecognition, donor dendritic cells in the allograft migrate to secondary lymphoid tissues, where they directly present allogeneic major histocompatibility complex (MHC) molecules to host T cells. Only CD8+ T cells recognizing donor class I MHC is shown, but CD4+ T cells can also directly recognize donor class II MHC.
In the case of indirect allorecognition, recipient dendritic cells that have entered the allograft, transport donor MHC proteins to secondary lymphoid tissues and present peptides derived from these MHC proteins to alloreactive host T cells.
This is shown for CD4+ T cells, and indirect recognition of allogeneic MHC by CD8+ T cells is likely less important. After both indirect and direct allorecognition, the T cells become activated and differentiate into effector CD4+ helper T cells and CD8+ cytotoxic T lymphocytes (CTLs).
The alloreactive effector T cells migrate into the allograft, become reactivated by alloantigen, and mediate damage. In the graft, direct recognition of allogeneic class I MHC by CD8+ CTLs is required for killing of graft parenchymal cells, because these cells express only allogeneic MHC.
In contrast, CD4+ helper T cells that can directly or indirectly recognize allogeneic class II MHC can be activated by donor or host antigen-presenting cells (APCs), respectively, and both can promote inflammation that damages the graft
Minor histocompatibility antigens can contribute to transplant rejection
Examples of minor histocompatibility antigens (mHA) include HA-1, HA-2, HB-1, and HY (sex-linked antigens). These are different versions of the same protein encoded by different alleles of a gene, leading to an immune response against the foreign version, such as in a male-to-female bone marrow transplant. Other examples include ACC-1, ACC-2, HA-8, and UTDP4-1, which are identified by the genes they originate from.
Mixed lymphocyte reaction
An in vitro model of T cells recognition of alloantigens
T cells from one individual are cultured with leukocytes of another and measure their responses
The magnitude of these responses is proportional to the extent of MHC differences and it is a rough predictor of outcomes of grafts exchanges between two individuals
Culturing mononuclear cells
Culturing mononuclear cells (MNCs) from one individual with MNCs to another individual. If the two individuals differ in MHC alleles, a large proportion of the lymphocytes proliferate in 4 to 7 days, and it is called allogenic MLR.
If cells from two MHC-disparate individuals are mixed, each can react against the other and both will proliferate, thus resulting in two-way MLR. To simplify this, one-way MLR is carried out where leukocyte populations from one individual are rendered in capable of proliferation by treating with anti-mitotix drug, mitomycin C or gamma-irradiation, and they act as stimulator cells and the untreated cells from another individual are capable of proliferation (responder cells). Among the T cells that respond to antigen, CD4 are specific for MHC II, and CD8 for MHC I. Because of the high frequency of T cells that can directly recognize allogenic MHC, responses to alloantigens are the only primary T cell responses.
Immune mechanisms of graft rejection
In hyperacute rejection, preformed antibodies reactive with vascular endothelium activate complement and trigger rapid intravascular thrombosis and necrosis of the vessel wall.
In acute rejection, CD4+ and CD8+ T lymphocytes reactive with alloantigens on endothelial cells and parenchymal cells mediate damage to these cell types. Alloreactive antibodies formed after engraftment may also contribute to parenchymal and vascular injury.
In chronic rejection with graft arteriosclerosis, injury to the vessel wall leads to intimal smooth muscle cell proliferation and luminal occlusion. This lesion may be caused by a chronic inflammatory reaction to alloantigens in the vessel wall.
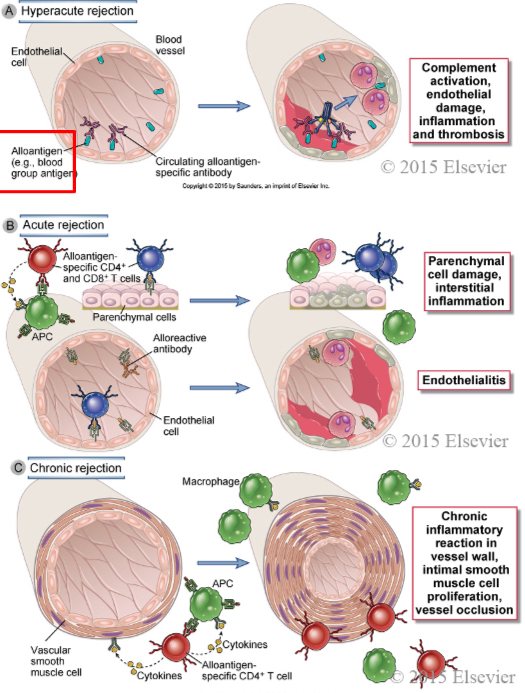
Graft rejection mediators
Within minutes: antibodies
Days to weeks: CTLs, CD4, antibodies
Months to years: mostly CD4 T cells
Hyperacute rejection
Occurs within minutes of transplantation; characterized by thrombosis of graft vessels and ischemic necrosis of the graft.
Antibodies (prior transfusions or reactions with alloantigens) bind to specific antigens on endothelial cells in the grafts and activate complement and clotting systems.
Acute rejection
Occurs within days or weeks after transplantation-a cause for early failures.
Mediated by T cells that react with alloantigens in the graft. They can be CTLs that destroy graft cells or the T cells that react with cells in graft vessels leading to vascular damage. Antibodies may also contribute.
Chronic rejection
Occurs over months or years and it leads to progressive loss of graft function. Manifested as fibrosis of the graft or gradual narrowing of graft vessels called graft arteriosclerosis.
Mostly CD4 T helper cells mediate graft rejection; they secrete cytokines/DTH reaction-stimulate fibroblasts and vascular smooth muscle cells in the graft
Thrombosis
Formation of a blood clot (partial or complete blockage) within blood vessels, whether venous or arterial, limiting the natural flow of blood and resulting in clinical sequela.
Bone marrow transplantation
Induction of graft-versus-host disease in dogs that have received a bone marrow allograft.
Donor marrow contains immunocompetent cells, the graft may reject the host causing Graft-Versus-Host disease (GVHD)
Additional notes on bone marrow transplantation
•Bone marrow transplantation: Some of the bone marrow of the recipient has to be destroyed (irradiation or chemotherapy with cyclophosphamide) to suppress the recipient’s immune system.
•Immune suppressive state of recipient makes graft rejections rare.
•But donor bone marrow contains immunocompetent cells, the graft may reject the host causing Graft-versus-host disease (GVHD).
•Affects mostly skin, liver and intestines.
•It develops as donor T cells recognize alloantigens on the host cells due to MHC disparity between graft and host.
•Careful MHC typing is critical.
GVHD: donor cells recognizing recipient’s antigens
Xenotransplantation
Hyperacute rejection is a major problem because the recipients often contain antibodies called “natural antibodies” of IgM isotype
Their production do not require prior exposure to the xenoantigens
Natural antibodies are produced against bacteria that normally inhabit the gut and the antibodies cross-react with cells of other species
Both innate and adaptive immune systems contribute to xenograft rejection
Immunosuppressive factors that prevent rejection of the fetus by the mother’s immune system
•Some of the immunosuppressive factors and processes that prevent rejection of the fetus by the mother's immune system.
•2, 3 dioxygenase-mediates catabolism of tryptophan-required for T cell growth
•No MHC’s on preimplanted embryos or oocytes and tropoblasts on maternal tissues
•Treg cells promote maternal-fetal tolerance
•Th2 cytokines favor pregnancy
•HLA-E molecules (nonpolymorphic class Ib molecules) prevent NK cell-mediated cytotoxicity
•uNK-uterine NK cells also called endometrial gland cells have been described in rodents, bats, pigs and horses. They promote fetal tolerance by suppressing Th17 responses by secreting IFN-gamma.
•IL-10 produced by Treg cells favor pregnancy
•CD95L (FAS ligand) expressed on the trophoblast tissues interacts with FAS on maternal T cells leading to their killing.
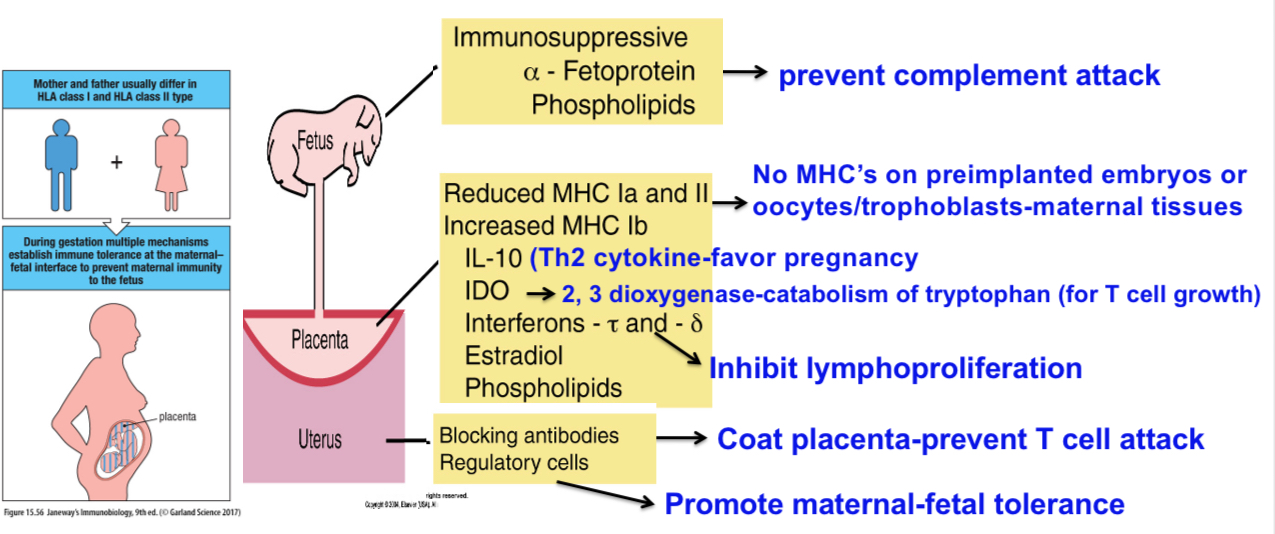
Blocking antibodies
Blocking antibodies are a type of antibody (primarily IgG-IgG4 and others?) that promotes maternal-fetal tolerance by shielding the fetus from the mother's immune system. They achieve this by binding to paternal HLA antigens on the fetal cells, and/or by binding to components of the maternal immune system, preventing it from recognizing the fetus as foreign and attacking it. They are crucial for a successful pregnancy, and a lack of them can lead to miscarriage or other pregnancy complications.
Treatments for graft rejection
Each major category of drugs used to prevent or to treat allograft rejection is shown along with the molecular targets of the drugs. APC, Antigen-presenting cell; CTL, cytotoxic T lymphocyte; IL, interleukin; mTOR, mechanistic target of rapamycin; TCR, T cell receptor.
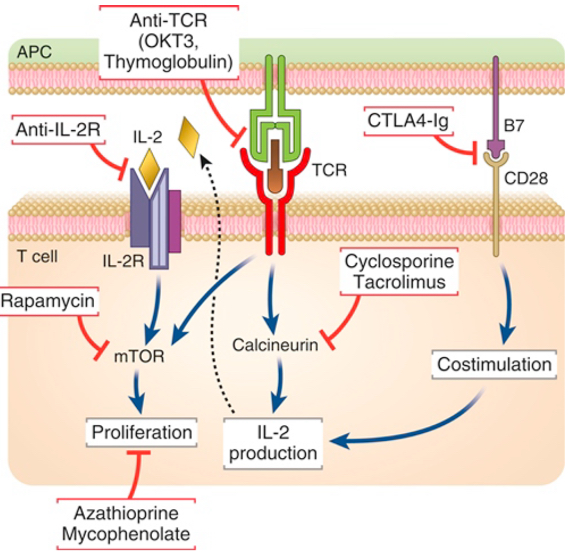
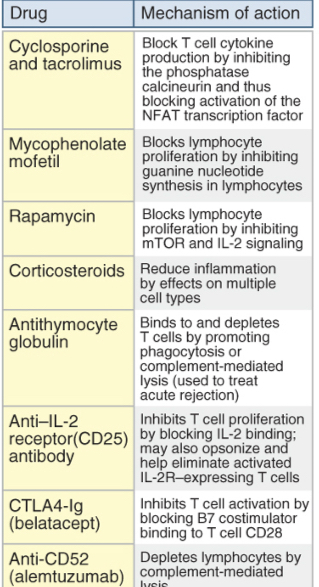
Table lists agents used to treat rejection of organ grafts and their mechanisms of action. Like cyclosporine, tacrolimus (FK506) is a calcineurin inhibitor, but it is not as widely used. CTLA4-Ig, Cytotoxic T lymphocyte–associated protein 4–immunoglobulin (fusion protein); IL, interleukin; NFAT, nuclear factor of activated T cells.
Prevention and Treatment of graft rejection
1.Immunosuppression: Inhibitors of T cell activation and their effector functions.
E.g. cyclosporine-block Calcineurin/NFAT pathway; inhibits cytokine genes in T cells.
Side effects: generalized non-specific immunosuppression-enhance susceptibility to intracellular microbes and increase in the incidence of tumors.
2. Immunologic tolerance: blockers of costimulators (CTLA4- Ig).
3. Strategies to induce the generation of Treg cells.