Chemistry 11 - Phase Changes & Kinetic Energy
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
What is a chemical change?
When a new substance is formed
What is a physical change?
A change is phase, no new substance formed
What is melting and freezing temperature?
The temperature a a solid changes to liquid and vice-versa.
They are opposites and the same temperature.
What is boiling and condensation temperature?
The temperature a liquid changes to a gas and vice versa.
They are opposites and the same temperature.
Explain the phase change graph
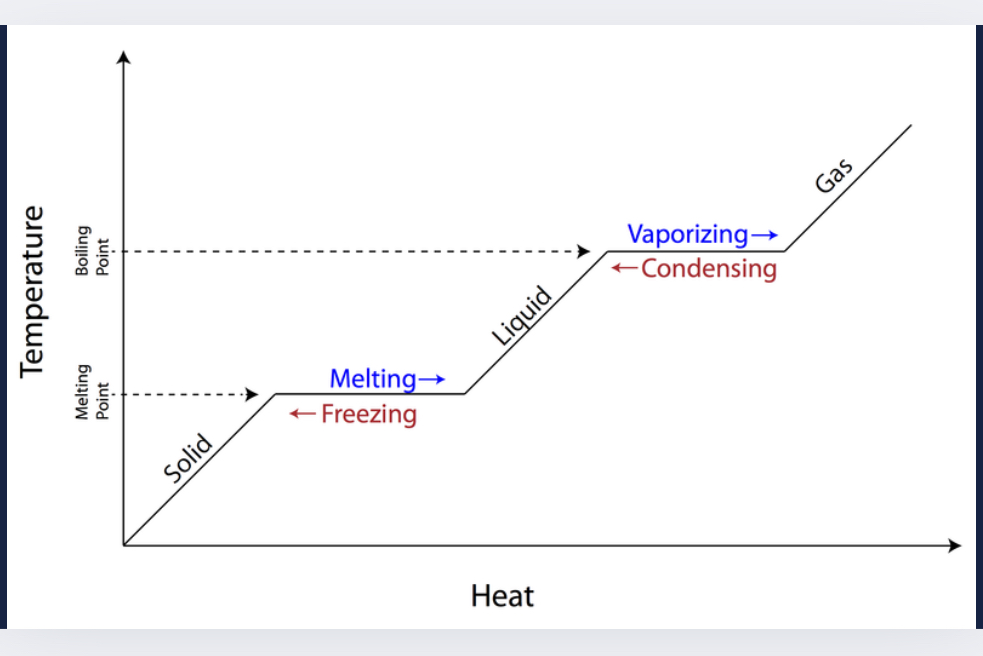
What is happening in the plateaus of a temp/heat graph?
Bonds are breaking and phases are changing.
What are the 3 kinds of kinetic energy?
rotational
translational
vibrational
What is rotational energy?
molecules rotate around their x,y,z axes of rotation
maintain their bond lengths and angles
What is vibrational energy?
bonds within molecules stretch and shrink or adjust their angles (vibrations)
What is translational energy?
molecules traveling in a straight line.
What is the kinetic energy at solid state?
increasing amounts of vibrational and translational energy
no rotational energy
What is the kinetic energy at melting point?
bonds are breaking, allowing rotational energy
What is the kinetic energy at liquid state?
increasing amounts of vibrational, translational and rotational energy
What is the kinetic energy at boiling point?
the translational energy is high enough to break bonds restricting movement - can move more
What is the kinetic energy at gas state?
increasing amounts of vibrational, rotational and translational energy.
What energy is the greatest and breaks bonds at every state?
Translational energy