Chapter 5: Allostery in Hemoglobin
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
102 Terms
The binding of a negative allosteric effector to an allosteric enzyme (K-system) that shows positive cooperativity in substrate binding:
a. decrease the cooperativity of the substrate.
b. increase the number of R conformations.
c. cause a shift to the right in the sigmoidal curve of v (velocity) versus [S].
d. cause a shift to the left in the sigmoidal curve of v (velocity) versus [S].
e. increase the affinity of binding S.
c. cause a shift to the right in the sigmoidal curve of v (velocity) versus [S].
explanation:
A negative allosteric effector (inhibitor) binds to an allosteric site and stabilizes the T (tense) state of the enzyme, which has lower affinity for the substrate.
In a K-system, the effector primarily affects the apparent K₀.₅ (substrate concentration giving half-maximal velocity) rather than Vmax.
As a result, more substrate is needed to reach the same velocity → the curve shifts to the right.
This also means the affinity for the substrate decreases, but the maximum velocity (Vmax) remains the same.
During the Monod-Wyman-Changeux (MWC) model, the ihibitor is also called ________.
negative effector
What is the effect of a negative allosteric effector (inhibitor)?
It binds preferentially to the T form, stabilizing it and shifting the equilibrium toward T₀.
How does an inhibitor affect substrate binding in the MWC model?
It decreases substrate affinity and increases the apparent cooperativity of substrate binding.
The addition of a negative allosteric effector to an enzyme that acts as a V-system causes the half-maximum velocity (K0.5) to ____ and Vmax to ____.
a. remain constant; remain constant
b. decrease; increase
c. remain constant; increase
d. decrease; remain constant
e. remain constant; decrease
(e) remain constant; decrease
What does the Monod-Wyman-Changeux (MWC) model describe?
It explains allosteric regulation, where enzymes exist in two interconvertible forms — R (relaxed) and T (tense) — and modulators shift the equilibrium between them.
What characterizes a K system in the MWC model?
The substrate concentration giving half-maximal binding (K₀.₅) changes with effectors, while Vmax remains constant.
What characterizes a V system in the MWC model?
The Vmax changes in response to allosteric effectors, but K₀.₅ remains constant.
How do the R and T forms differ in the MWC model?
They have the same affinity for substrate (S) but different affinities for activators (A) and inhibitors (I) and different catalytic activities.
Under what conditions is MWC regulation especially important?
When the cellular concentration of substrate (S) is much greater than K₀.₅, ensuring that activity depends mainly on the enzyme’s state (R vs. T).
Myoglobin and Hemoglobin are a wonderful example of _________
cooperativity and allosteric regulation
___________ and __________ are oxygen- transport and oxygen-storage proteins, respectively
Hemoglobin (blood) and myoglobin (muscle)
Is myoglobin monomeric or tetrameric?
monomeric
Is hemoglobin monomeric or tetrameric?
tetrameric
What is the amino acid composition and molecular weight of myoglobin?
Myoglobin has 153 amino acids and a molecular weight of ~17,200 Da.
Describe the subunit composition of hemoglobin.
Two α chains (141 residues each)
Two β chains (146 residues each).
What structural feature is shared by both hemoglobin and myoglobin?
Both have 8 α-helices, labeled A through H, and each subunit contains a heme group that binds oxygen.
What type of oxygen-binding curve does myoglobin display?
A hyperbolic curve, similar to a Michaelis–Menten binding curve.
What type of oxygen-binding curve does hemoglobin display?
A sigmoidal (S-shaped) curve, indicating cooperative oxygen binding.
Why does myoglobin follow a hyperbolic curve?
Because myoglobin is monomeric, it has a single binding site for oxygen — no cooperativity.
Why does hemoglobin show a sigmoidal oxygen-binding curve?
Hemoglobin is tetrameric, and oxygen binding to one subunit increases the affinity of the others — positive cooperativity.
Mb and Hb use heme to _____, allowing ______.
bind Fe2+, allowing oxygen binding and transport.
What structural components make up the heme group?
4 pyrrole rings.
Iron interacts with _____ ligands.
Four of these are the ___________.
Iron interacts with five/six ligands
Four of these are the N atoms of the porphyrin
What is the fifth ligand to the heme iron in myoglobin? Why is the residue called His F8?
The imidazole side chain of His F8.
It is the 8th residue on the 6th (F) helix.
What happens when myoglobin or hemoglobin binds oxygen?
The O₂ molecule becomes the sixth ligand bound to the heme iron.
How is the O₂ molecule positioned when bound to heme?
The O₂ is tilted relative to a perpendicular to the heme plane.
O2 Binding Alters _________ _________.
Mb Conformation
In deoxymyoglobin, the ferrous ion lies ______ ______ the plane of the heme
In deoxymyoglobin, the ferrous ion lies 0.055 nm above the plane of the heme
What happens to the heme iron when oxygen binds to myoglobin?
The heme Fe is drawn toward the plane of the porphyrin ring.
How far above the heme plane is Fe²⁺ after O₂ binding?
Only 0.026 nm above the plane.
What is the effect of this conformational change in myoglobin (Mb)?
The change has very little consequence for Mb’s structure
What is the effect of a similar conformational change in hemoglobin (Hb)?
It initiates conformational changes that are transmitted to adjacent subunits, leading to cooperatively.
What type of protein is myoglobin (Mb)?
A monomeric heme protein.
Mb polypeptide __________ the heme group
Mb polypeptide "cradles" the heme group
Fe in Mb is ________ - the form that binds oxygen
Fe2+ - ferrous iron
Oxidation of Fe yields ______(______ iron) charge
Fe 3+ - ferric iron
Mb with Fe3+ is called __________ and ___________ bind oxygen
Mb with Fe3+ is called metmyglobin and does not bind oxygen
Hb Has an _______ Tetrameric Structure
α2β2
Which subunit contacts are important for subunit packing in hemoglobin?
The α₁β₁ and α₂β₂ contacts, involving helices B, G, H, and the GH corner.
Do the α₁β₁ and α₂β₂ contacts change upon oxygenation?
No, they remain unchanged upon oxygen binding.
Which hemoglobin contacts are referred to as “sliding contacts”?
The α₁β₂ and α₂β₁ interfaces.
What happens at the “sliding contacts” during oxygenation?
These α₁β₂ and α₂β₁ contacts shift, allowing the conformational change that drives cooperative oxygen binding.
Is this the O2-Binding Curves of Mb or Hb?
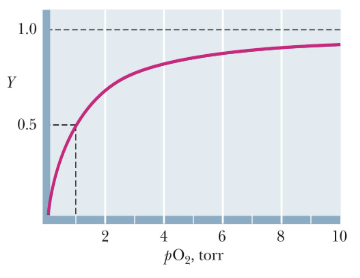
Mb
Is this the O2-Binding Curves of Mb or Hb?
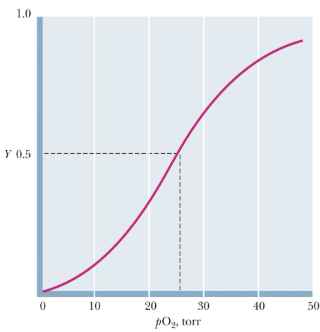
Hb
What shape does hemoglobin’s O₂ binding curve display? What does this indicate?
A sigmoid (S-shaped) curve. Cooperativity in oxygen binding — binding of one O₂ increases the affinity for the next.
How many heme groups does hemoglobin have?
Four heme groups.
Is hemoglobin’s cooperativity caused by direct heme-heme interactions?
No, the heme groups are far apart, so cooperatively arises from conformational changes transmitted through the protein, not direct heme contact.
In the O₂-binding curve for Hb:
the experimentally observed value for n is _______;
the hypothetical curve if n = ______ shows perfect cooperativity,
and if n = ______ the sites are non-interacting.
K is the ________ constant.
2.8; 4; 1; dissociation
Movement of the Fe²⁺ atom by less than ______ nm upon __________ leads to changes in the position of the ______ ______ atom, which cause __________ changes in the __________ molecule.
0.04; oxygenation; heme iron; conformational; hemoglobin
During oxygen binding to Hb, a ______° rotation of one ______ pair occurs.
During oxygen binding to Hb, a 15° rotation of one αβ pair occurs.
In deoxy-Hb, the ______ atom lies out of the heme plane by about ______ nm.
In deoxy-Hb, the iron atom lies out of the heme plane by about 0.06 nm.
Upon O₂ binding, the ______ atom moves about ______ nm closer to the plane of the heme.
Upon O₂ binding, the Fe²⁺ atom moves about 0.039 nm closer to the plane of the heme.
It is as if the ______ is drawing the ______ ______ into the plane.
It is as if the O₂ is drawing the heme iron into the plane.
As Fe²⁺ moves, it drags ______ and the ______ helix with it (also the ______ and ______ corners).
As Fe²⁺ moves, it drags His F8 and the F helix with it (also the EF and FG corners).
This change is transmitted to the ______ interfaces, where conformational changes lead to the rupture of ______ ______.
This change is transmitted to the subunit interfaces, where conformational changes lead to the rupture of salt bridges.
Salt bridges that stabilize deoxy-Hb are broken in ________.
oxy-Hb
Salt bridges are present between different subunits in human deoxy-Hb. How many salt bridges stabilize the T state, and what happens to them during oxygenation?
In human deoxy-Hb, the deoxy (T) state is stabilized by 8 salt bridges, which are broken in the transition to the oxy (R) state.
How do the salt bridge and H-bond interactions differ between the α- and β-chains in deoxy-Hb?
In deoxy-Hb, the α-chains form salt bridges and H-bonds involving both N-terminal and C-terminal residues, while the β-chains form salt bridges and H-bonds involving only their C-terminal residues.
What happens when oxygen first begins binding to hemoglobin?
Oxygen binds first to the two α subunits of hemoglobin.
When does the large conformational change in hemoglobin occur during oxygen binding? What does that lead to?
The large conformational change occurs when two oxygens are bound to Hb, which increases the affinity of the β subunits for oxygen.
How does the oxygen affinity of deoxy-Hb compare to oxy-Hb?
Deoxy-Hb has a low affinity for oxygen.
Why do the β subunits of deoxy-Hb have low oxygen affinity initially?
In deoxy-Hb, the heme groups of the β subunits are inaccessible to oxygen.
Which subunit does oxygen bind to first, and what effect does this have?
The first oxygen binds to an α subunit, causing small changes in the tertiary structure of the other α subunit, which increase its affinity for oxygen about threefold (KNF model, sequential)
Which models best feature Cooperatively in Hb?
Cooperativity in hemoglobin has features of both the concerted (MWC) model and the sequential (KNF) model.
Oxygen first binds to the α subunits, causing small tertiary structural changes (KNF model), and when two oxygens are bound, a large conformational change (MWC model) occurs that increases the β subunits’ affinity for oxygen.
Which subunits does oxygen bind to first in hemoglobin?
Oxygen first binds to the α subunits (α α).
Hb with two oxygens bound is in _________ with a form of the protein in which all four subunits are ___________.
Hb with two oxygens bound is in equilibrium with a form of the protein in which all four subunits are in the R state
What model describes the transition from the T to the R state in hemoglobin?
This equilibrium between the two-oxygen-bound form and the all-R-state form is equivalent to the T/R transition in the MWC model (symmetric, where it all happens at once. NOT sequential like KNF)
When does the large change in hemoglobin’s quaternary structure occur?
the T/R transition
Cooperative Binding of _______ Influences Hemoglobin Function
Oxygen
Mb, an _______-storage protein, has a greater _______ for oxygen at all oxygen pressures
Mb, an oxygen-storage protein, has a greater affinity for oxygen at all oxygen pressures
Why must hemoglobin have a different oxygen-binding behavior than myoglobin?
it must bind oxygen in lungs and release it in capillaries
Hb becomes saturated with O2 in the lungs, where the partial pressure of O2 is about _________ torr
100 torr
What is the partial pressure of oxygen in the capillaries, and what happens to Hb there?
In the capillaries, the pO₂ is about 40 torr, and oxygen is released from hemoglobin.
What is perfect for hemoglobin function?
cooperative binding
H+ Promotes _____________ from Hemoglobin
Dissociation of Oxygen
What is the Bohr Effect?
The Antagonism of O2 Binding by H+ is Termed the Bohr Effect
Binding of protons diminishes ___________.
Binding of oxygen diminishes ___________.
Binding of protons diminishes oxygen binding
Binding of oxygen diminishes proton binding
Protonation of __________ induces R-to-T transition of hemoglobin, __________ affinity for oxygen.
Protonation of histidine 146 induces R-to-T transition of hemoglobin, decreasing affinity for oxygen.
In deoxyhemoglobin _______ amino acid residues form _________ that stabilize the ________ quaternary structure.
In deoxyhemoglobin three amino acid residues form two salt bridges that stabilize the T quaternary structure.
In condition of alkalosis (serum pH higher than normal - > 7.45) :
A - affinity of O2 for hemoglobin is higher. The curve will shift to the left
B - affinity of O2 for hemoglobin is higher. The curve will shift to the right
C - O2 saturation of hemoglobin will decrease at a given partial pressure of O2
D – None of the above
A – affinity of O₂ for hemoglobin is higher. The curve will shift to the left.
________ Promotes the Dissociation of O2 from Hemoglobin
CO2
___________ diminishes oxygen binding
Carbon dioxide
Hydration of CO2 in tissues and extremities leads to _________
proton production
CO2 + H2O ⇄ H+ + HCO3–
What happens to the protons produced from CO₂ hydration in the tissues?
taken up by hemoglobin as oxygen dissociates
What happens to hemoglobin and carbon dioxide in the lungs?
In the lungs, the reverse of the tissue reaction occurs — oxygen binds to hemoglobin, protons are released, and carbon dioxide is reformed and exhaled.
What happens at the tissue–capillary interface that affects oxygen binding by hemoglobin?
CO₂ hydration and glycolysis produce extra H⁺, promoting additional dissociation of O₂ from hemoglobin where oxygen is needed most.
What happens at the lung–artery interface that influences oxygen and carbon dioxide exchange?
Bicarbonate dehydration, required for CO₂ exhalation, consumes extra H⁺, promoting CO₂ release and O₂ binding to hemoglobin.
How does CO₂ favor oxygen release from hemoglobin?
By stabilizing the deoxyhemoglobin (T) state through formation of carbamate groups at the terminal amino residues, which are negatively charged and participate in salt bridges.
__________ stabilizes deoxyhemoglobin.
Carbamate group
What is 2,3-bisphosphoglycerate (2,3-BPG) and where is it found?
2,3-bisphosphoglycerate is a negative allosteric effector of hemoglobin found in red blood cells.
How does oxygen binding to hemoglobin change in the absence of 2,3-BPG?
In the absence of 2,3-BPG, oxygen binding to hemoglobin follows a rectangular hyperbola.
Under what condition does hemoglobin display a sigmoid oxygen-binding curve?
The sigmoid oxygen-binding curve is only observed in the presence of 2,3-BPG.
Where does 2,3-BPG bind on hemoglobin?
2,3-BPG binds at a site distant from the iron (Fe) atom where oxygen binds.
BPG lies at the _________.
BPG binding stabilizes the ___________ reducing the ___________.
BPG lies at the center of the cavity between the two β-subunits
BPG binding stabilizes the deoxy form (T state) reducing the affinity for oxygen
What interactions stabilize BPG binding within hemoglobin?
Negative charges interact with 8 positive charges in the cavity: 2 Lys, 4 His, 2 N-termini
Fetal Hemoglobin Has a _______ Affinity for O2
Higher
The fetus depends on its mother for O2, but its ______ is entirely independent
Gas exchange takes place across the ______
The fetus depends on its mother for O2, but its circulatory system is entirely independent
Gas exchange takes place across the placenta
Fetal hemoglobin has _____ affinity for 2,3 BPG. As a result _______.
lower
As a result, fetal Hb has a higher affinity for O2
Fetal Hb differs from adult Hb how?
γ-chains in place of β-chains giving α2γ2 structure