i) The role of haemoglobin in transporting oxygen and carbon dioxide
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Transport of oxygen
Oxygen is transported in erythrocytes bound to the haem groups in haemoglobin
Each molecule of haemoglobin contains four haem groups, each able to bond with one molecule of oxygen
Oxygen + Haemoglobin ⇌ Oxyhaemoglobin
In lungs: Hb + 4O₂ → HbO₈
In tissues: HbO₈ → Hb + 4O₂
Where does oxygen bind to?
It binds to the haem prosthetic group Fe²⁺
How many molecules of oxygen can haemoglobin carry?
4 oxygen molecules, 8 oxygen atoms in total
What is the partial pressure of oxygen?
The measure of oxygen concentration
The greater the partial pressure, the higher its concentration
What is cooperative binding?
The first oxygen molecule results in a conformational change in the structure of the haemoglobin molecule
This makes it easier for each successive oxygen molecule to bind
Transport of Carbon Dioxide
Waste CO₂ produced during respiration diffuses from the tissues into the blood
Name the ways CO₂ is transported around the body
5% of CO₂ dissolves directly in the blood plasma and is transported in solution
10% of CO₂ bind to haemoglobin, forming carbaminohaemoglobin
85% of CO₂ is transported in the form of hydrogen carbonate ions (HCO₃⁻)
85% of CO₂ is transported in the form of hydrogen carbonate ions (HCO₃⁻)
Describe that process:
CO₂ diffuses from the plasma into red blood cells
Inside red blood cells, CO₂ combines with water to form carbonic acid (H₂CO₃)
Carbonic acid dissociates readily into H⁺ and HCO₃⁻ ions
HCO₃⁻ ions diffuse out of the red blood cells and into the blood plasma, where they are transported in solution
What happens to the H⁺ ions formed during the dissociation of carbonic acid?
The H⁺ ions can combine with haemoglobin, forming haemoglobinic acid (HHb)
This prevents the H⁺ ions from lowering the pH of the red blood cell
In this situation, haemoglobin acting as a buffer
Why does oxyhaemoglobin dissociate under the influence of H⁺ ions?
More haemoglobin is needed to act as a buffer due to more H⁺ ions, so HbO₄ dissociate and the oxygen is released into the blood plasma.
Why is carbonic acid formed inside red blood cells?
Red blood cells contain the enzyme "carbonic anhydrase", which catalyses the reaction between CO₂ and water
The plasma contains very little carbonic anhydrase so H₂CO₃ forms slowly in plasma than in the cytoplasm of red blood cells
What is the chloride shift?
The movement of chloride ions into red blood cells that occurs when HCO₃⁻ ions are formed
How do negatively charged HCO₃⁻ ions move out of the cell?
Via a transport protein in the membrane
How do you prevent an electrical imbalance when the negatively charged HCO₃⁻ ions are moving OUT of the red blood cell?
Negatively charged chloride ions are transported into the red blood cells via the same transport protein
What would happen if chloride ions didn't move into the cell?
Red blood cells would become positively charged as a result of a build-up of hydrogen ions formed from the dissociation of carbonic acid.
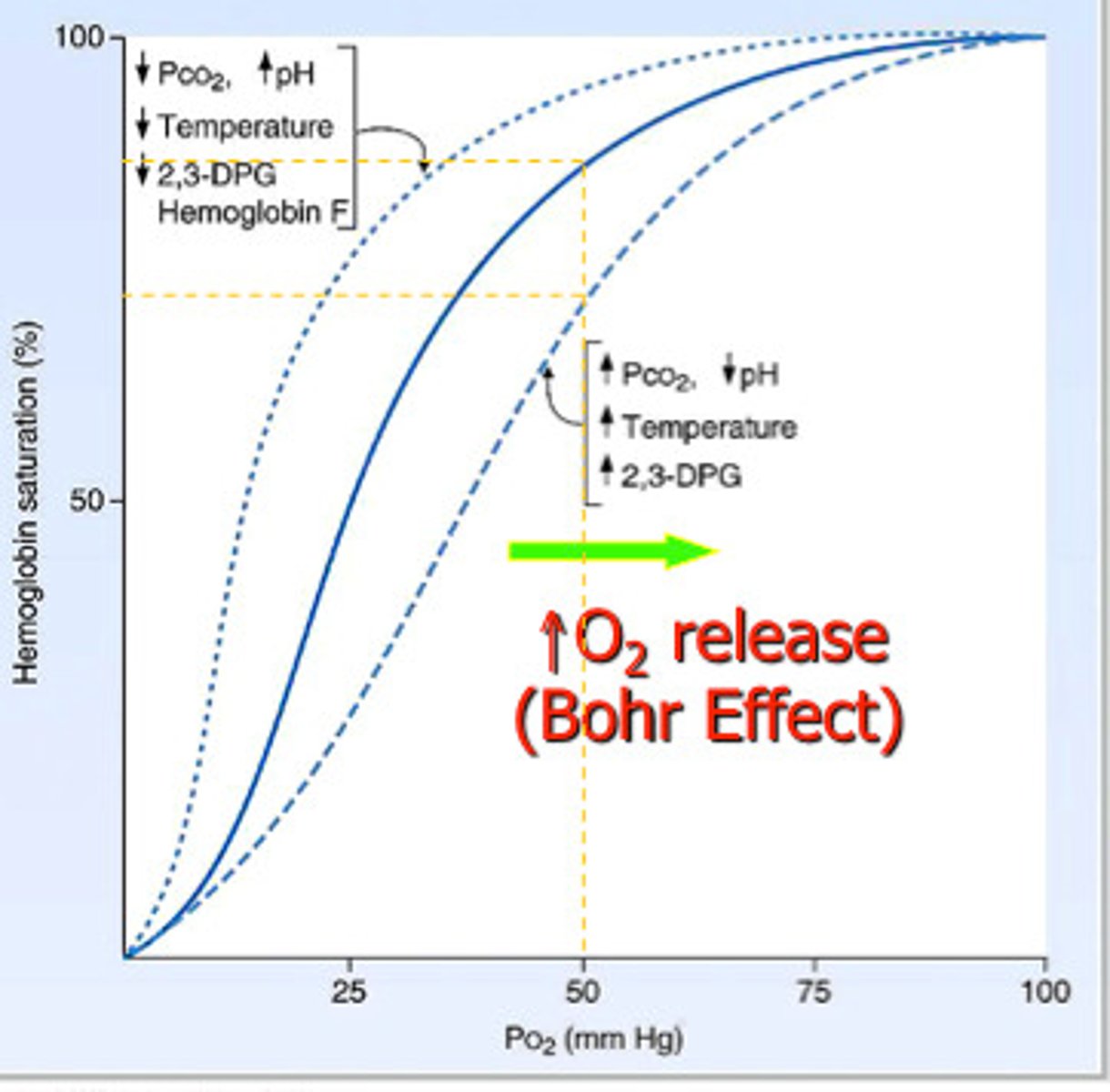
Bohr Effect
At high concs of CO₂, the shape of the Hb is altered.
Hb's affinity for oxygen is reduced, and there is more dissociation of oxyhaemoglobin, therefore more oxygen available for respiring tissues.
Hb acts as a buffer by binding to the H+ ions, forming haemoglobinic acid.
Hb binds to CO2 to form carbaminohaemoglobin.