ISU - Organic Chem Families and Characteristics
0.0(0)
Card Sorting
1/35
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
1
New cards
Functional group of __**amines**__
R-NH2
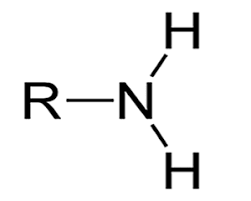
2
New cards
Functional group of __**amides**__
O=C-N-R-R
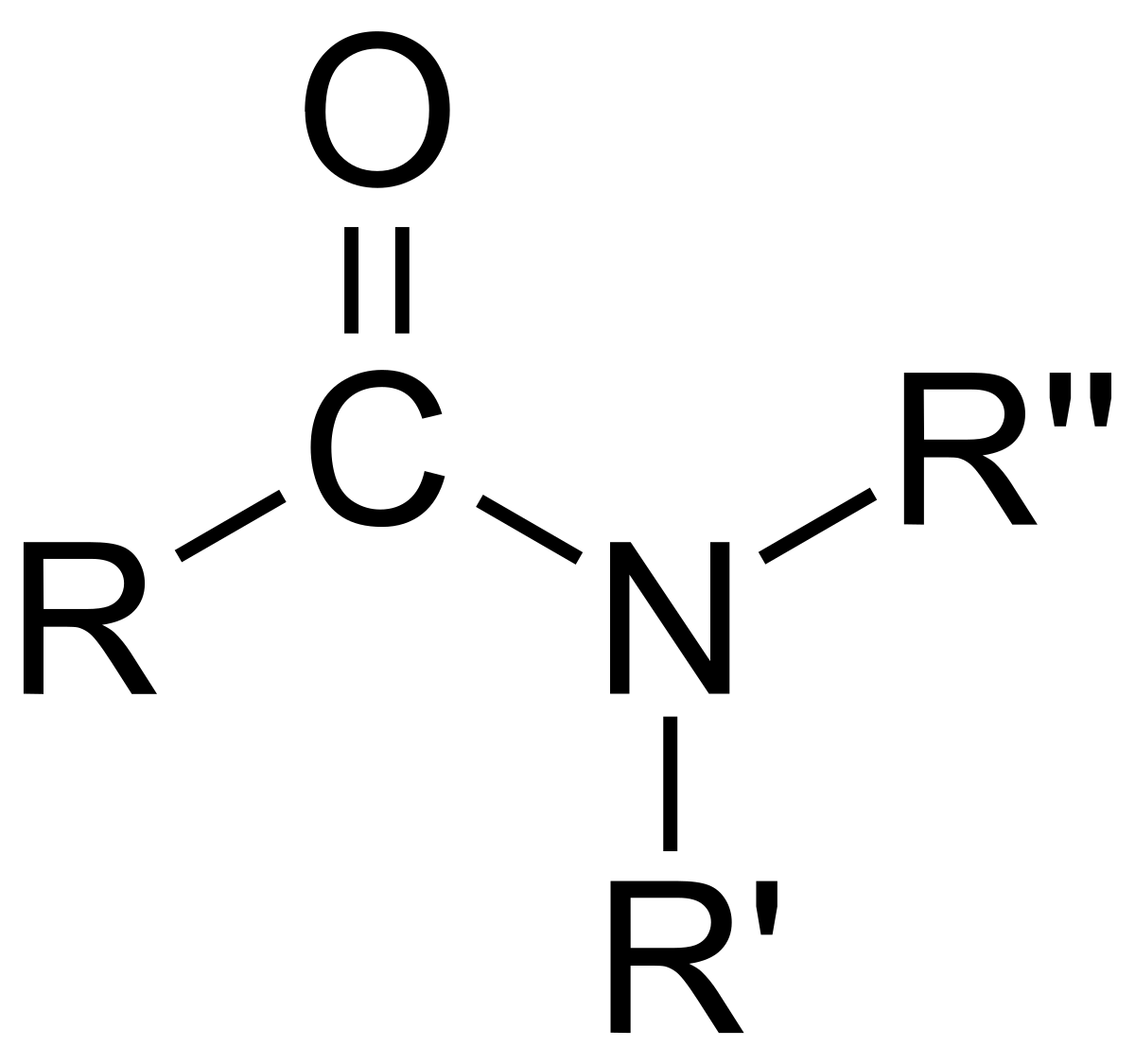
3
New cards
Functional group of __**aldehydes**__
R-C=O
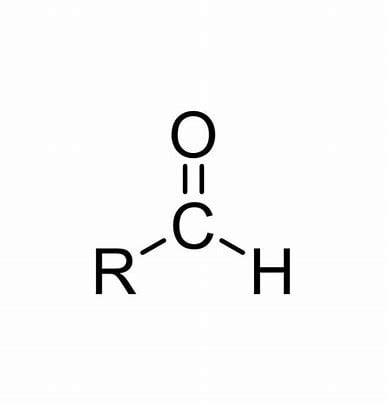
4
New cards
Functional group of __**ketones**__
C-C=O-C
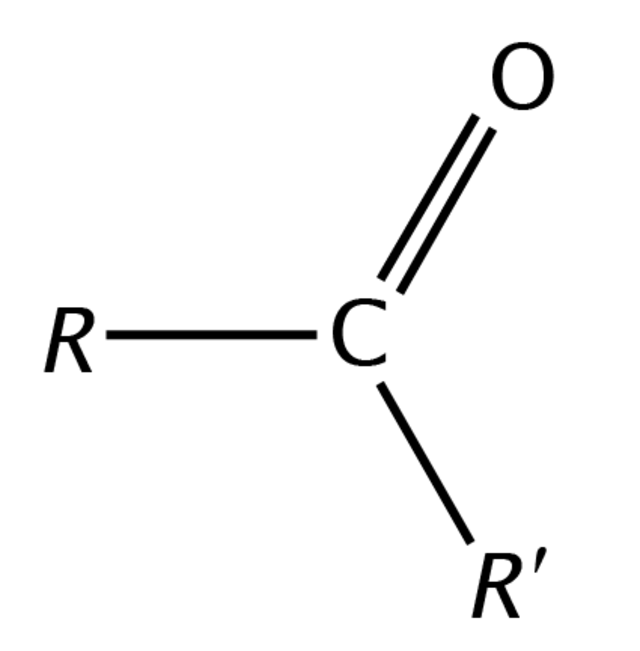
5
New cards
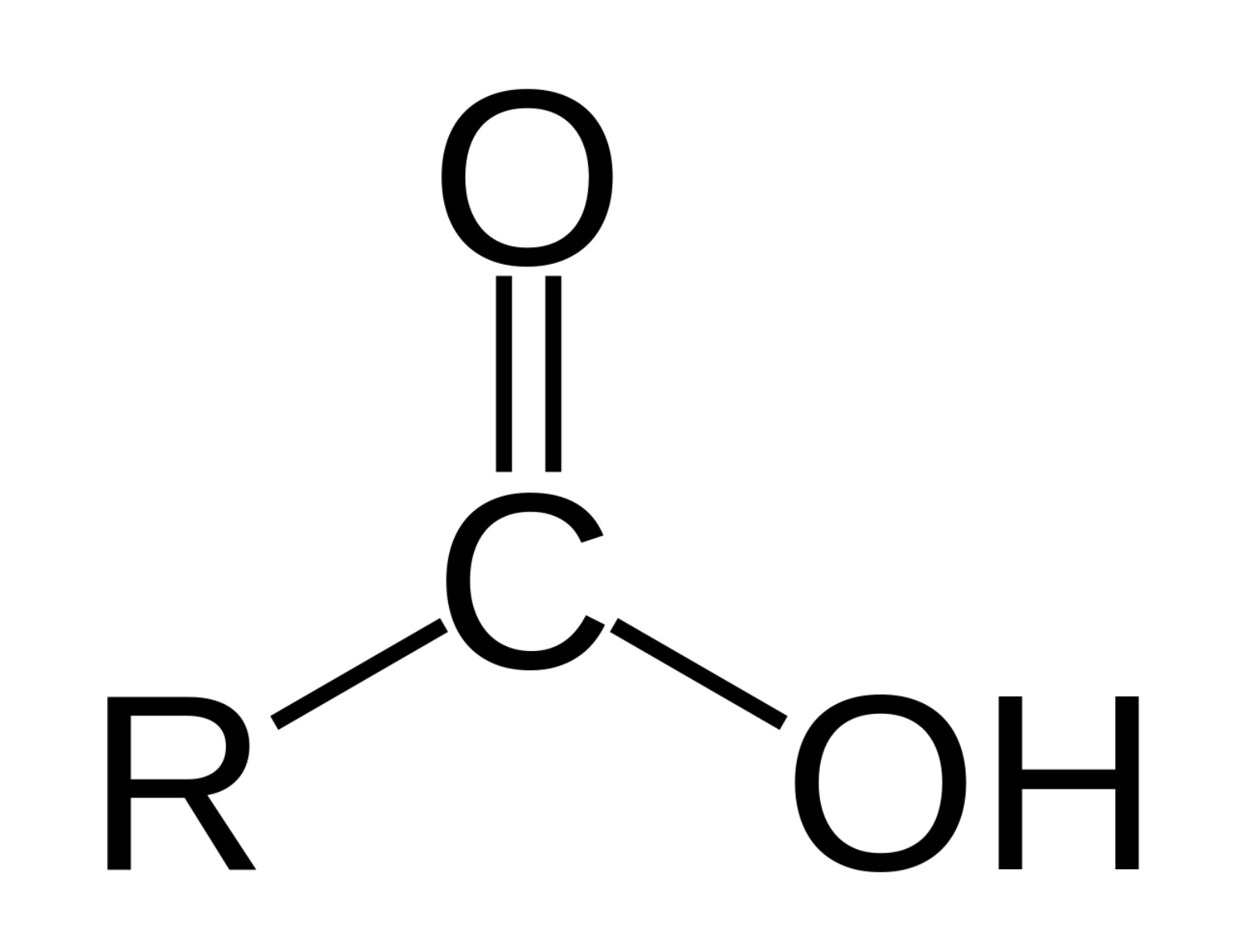
Functional group of __**carboxylic acids**__
R-C=O-OH
6
New cards
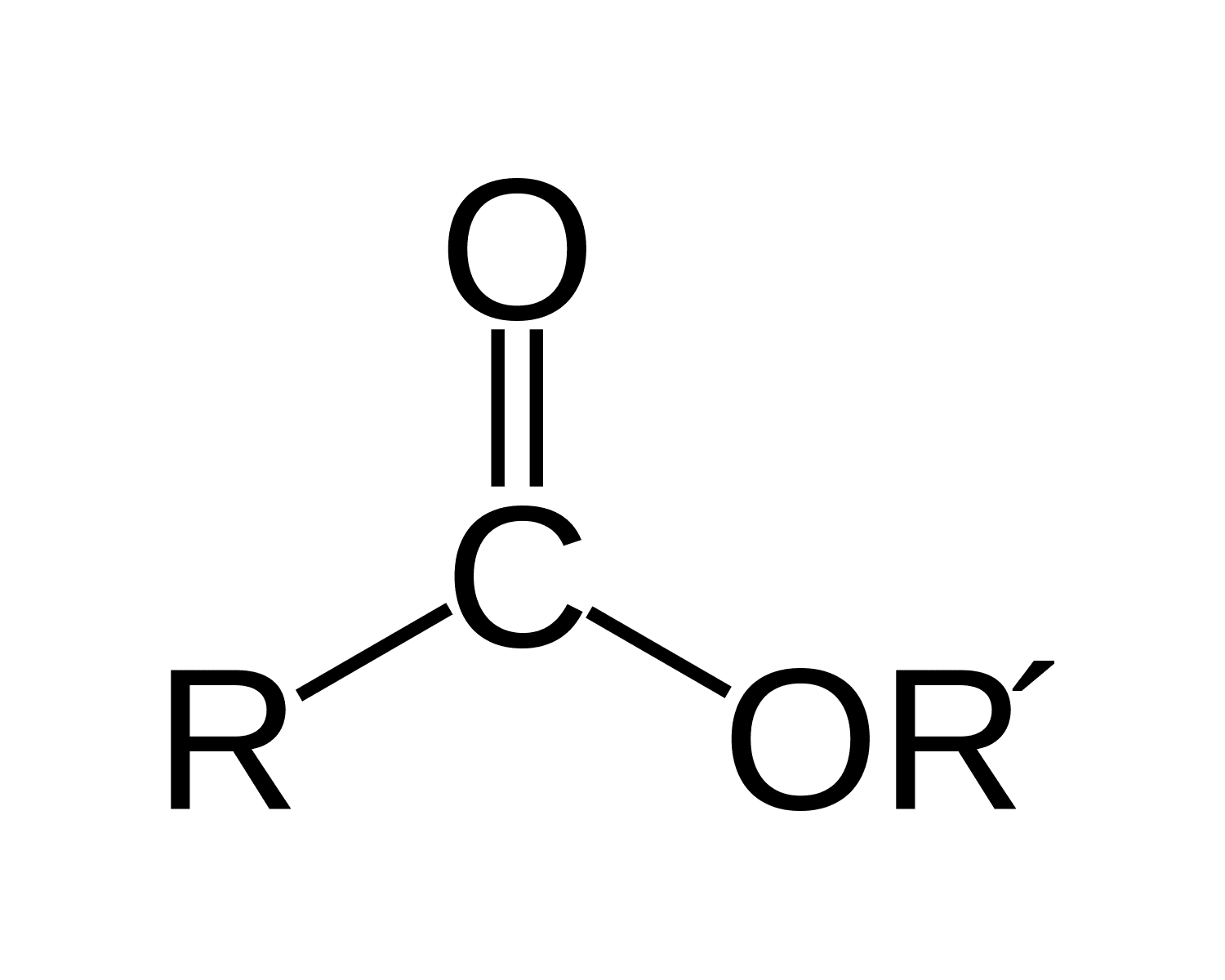
Functional group of __**Esters**__
R-C=O-O-R
7
New cards
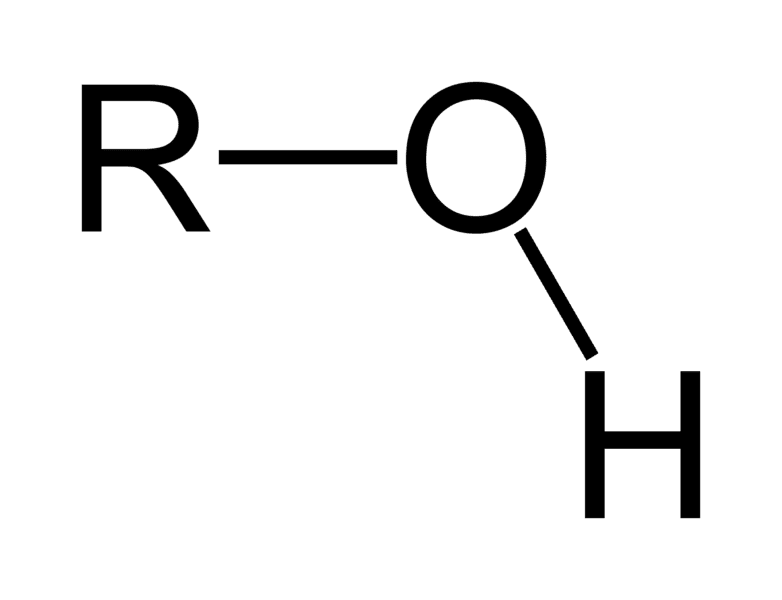
Functional group of __**alcohols**__
O-H
8
New cards
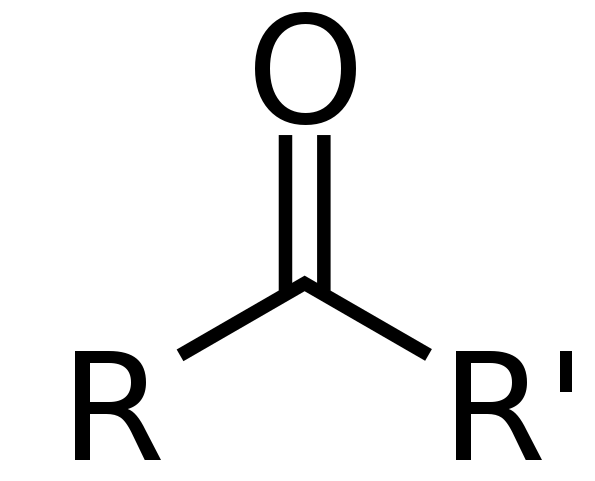
Functional group of __**ethers**__
R-O-R
9
New cards
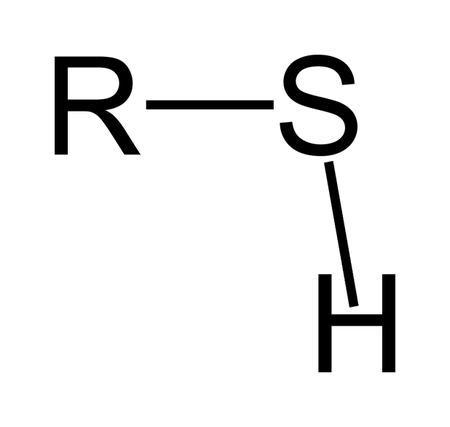
Functional group of __**thiols**__
R-S-R
10
New cards
What is the suffix for __**amines**__?
amine
11
New cards
What is the suffix for __**amides**__?
amide
12
New cards
What is the suffix for __**aldehudes**__?
al
13
New cards
What is the suffix for __**ketones**__?
one
14
New cards
What is the suffix for __**carboxylic acids**__?
oic acid
15
New cards
What is the suffix for __**esters**__?
yl, oate
16
New cards
What is the suffix for __**alcohols**__?
ol
diol
triol
diol
triol
17
New cards
What is the suffix for __**ethers**__?
ether
longer sub oxy
longer sub oxy
18
New cards
What is the suffix for __**thiols**__?
thiol
19
New cards
How do you simply name __**amines**__?
substituents + root-amine
20
New cards
How do you simply name __**amides**__?
substituents + acid root-amide
21
New cards
How do you simply name __**aldehydes**__?
subs - parent - locate C=O
22
New cards
How do you simply name __**ketones**__?
subs - parent - locate C=O
23
New cards
How do you simply name __**carboxylic acids**__?
location of functional takes precedence in numbering over other bonds
number where functional is located
subs - parent/bonds in chain - oic acid
number where functional is located
subs - parent/bonds in chain - oic acid
24
New cards
How do you simply name __**esters**__?
find alcohol
alcohol - yl - carboxylic acid - oate
alcohol - yl - carboxylic acid - oate
25
New cards
How do you simply name __**alcohols**__?
subs- parent-locate and number of functional - suffix
26
New cards
How do you simply name __**ethers**__?
subs, parent, root ether orshorter sub oxy, then ether
27
New cards
How do you simply name __**thiols**__?
subs- parent-thiol
28
New cards
How do intermolecular forces affect __**amines**__ polarity, solubility?
* Primary amines are more polar than others
* Hydrogen bonding creates the polarity
* As solubility decreases, chain length increases
* Hydrogen bonding creates the polarity
* As solubility decreases, chain length increases
29
New cards
How do intermolecular forces affect __**amides**__ polarity?
* Primary and secondary amides are more polar than tertiary
* Hydrogen bonding creates the polarity
* Tertiary cannot create H bonds
* Hydrogen bonding creates the polarity
* Tertiary cannot create H bonds
30
New cards
How do intermolecular forces affect __**aldehydes**__ polarity?
* H bonding increases boiling point
* Slightly less polar than ketones
* Slightly less polar than ketones
31
New cards
How do intermolecular forces affect __**ketones**__ polarity?
* H bonding increases boiling point
* Slightly more polar than aldehydes
* Slightly more polar than aldehydes
32
New cards
How do intermolecular forces affect __**carboxylic acid**__ polarity?
* Polar due to O atom
* Soluable
* Soluable
33
New cards
How do intermolecular forces affect __**esters**__ polarity?
* Polar
* Lower bp that carboxylic acids2
* Lower bp that carboxylic acids2
34
New cards
How do intermolecular forces affect __**alcohols**__ polarity?
* Polar
* Soluable
* high bp
* e- OH
* Soluable
* high bp
* e- OH
35
New cards
How do intermolecular forces affect __**ethers**__ polarity?
* weak polarity due to C-O
36
New cards
How do intermolecular forces affect __**thiols**__ polarity?
* less polar than alcohols