Enthalpy Change of Physical Processes, Calorimetry: Physical Chemistry
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Variation of enthalpy with temperature
Remember Constant-Pressure heat capacity:
Cp=(q/∆T) → q= Cp∆T= nCpm∆T
If we know the heat capacity of the system, we can calculate the change of the enthalpy with temperature.
∆H=nCpm∆T
Enthalpy is also a state function. This formula is true for any process, not just the isobaric process.
Enthalpy of an ideal gas:
Cpm=Cvm+R
For an ideal gas only (He, Ar): Cvm=(3/2)R → Cpm= (5/2)R
Enthalpy Whiteboard Breakdown

Enthalpy change of phase transitions
The standard state of a substance is the pure substance at exactly 1 bar.
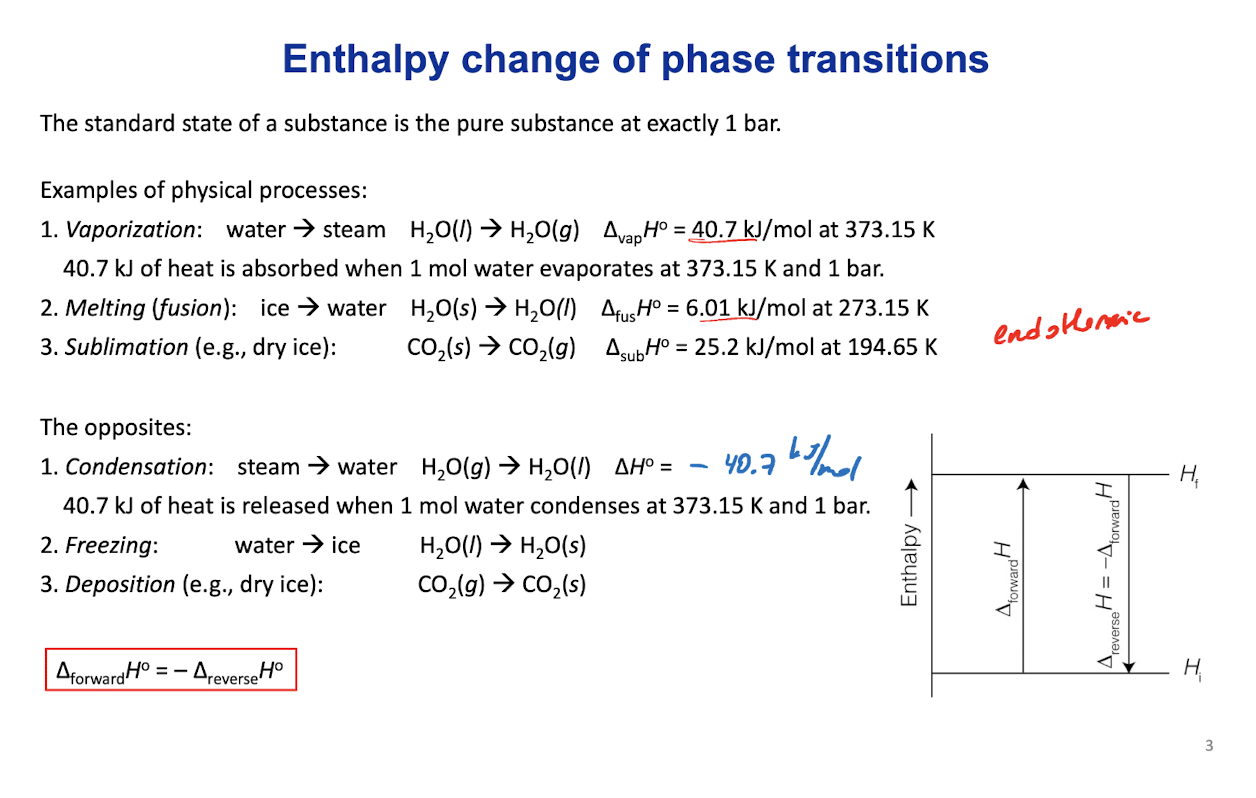
How do ∆fusH˚ and ∆vapH˚ relate to the molecular structure?
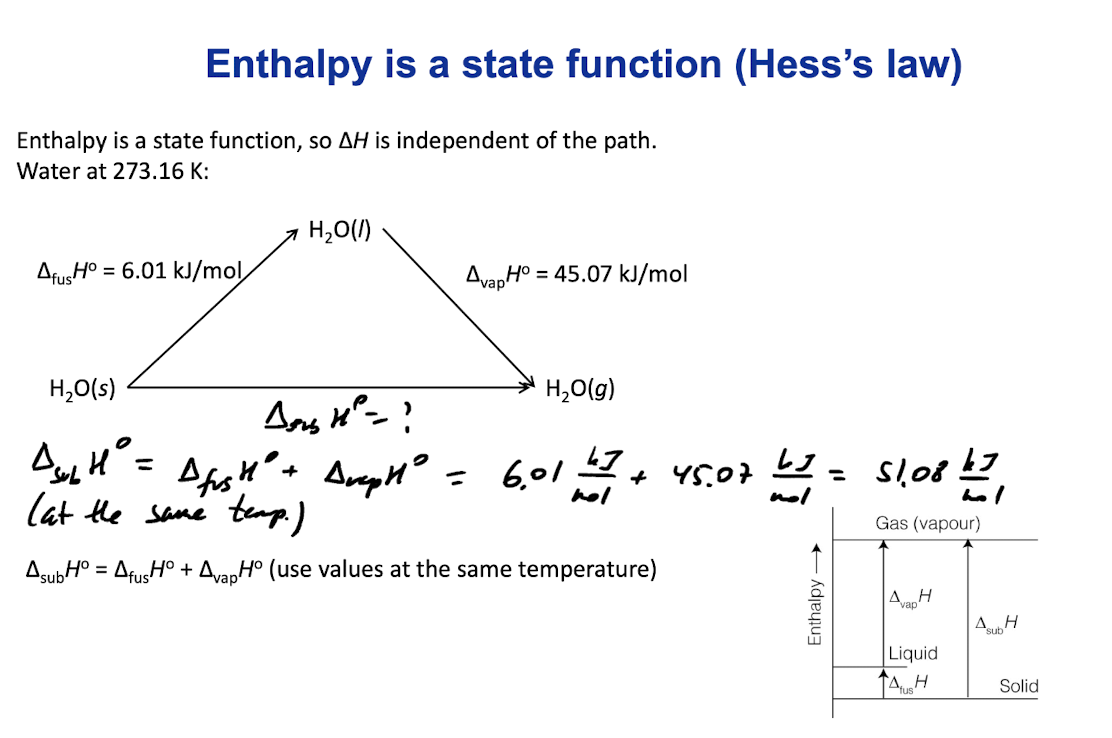
Hess’s Law
Enthalpy is a state function, so ∆H is independent of the path.
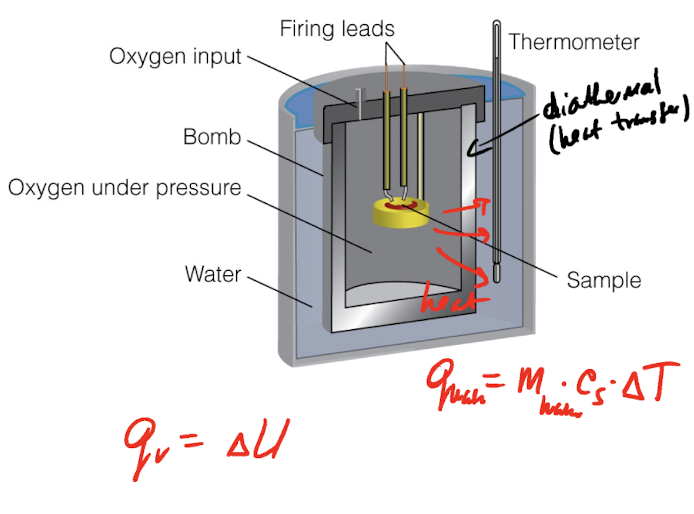
Bomb Calorimeters
Constant V
A calorimeter is a device for measuring energy transferred as heat.
Constant-volume bomb calorimeters (qv=∆U)
Run a reaction and measure the temperature change (∆T) in the surrounding water bath.
q=C∆T C= calorimeter constant
Calibration of the calorimeter:
(a) measure ∆T of a known reactionl
(b) pass electric current “I” from a voltage source V over time t to generate Joule heat:
q=IVt
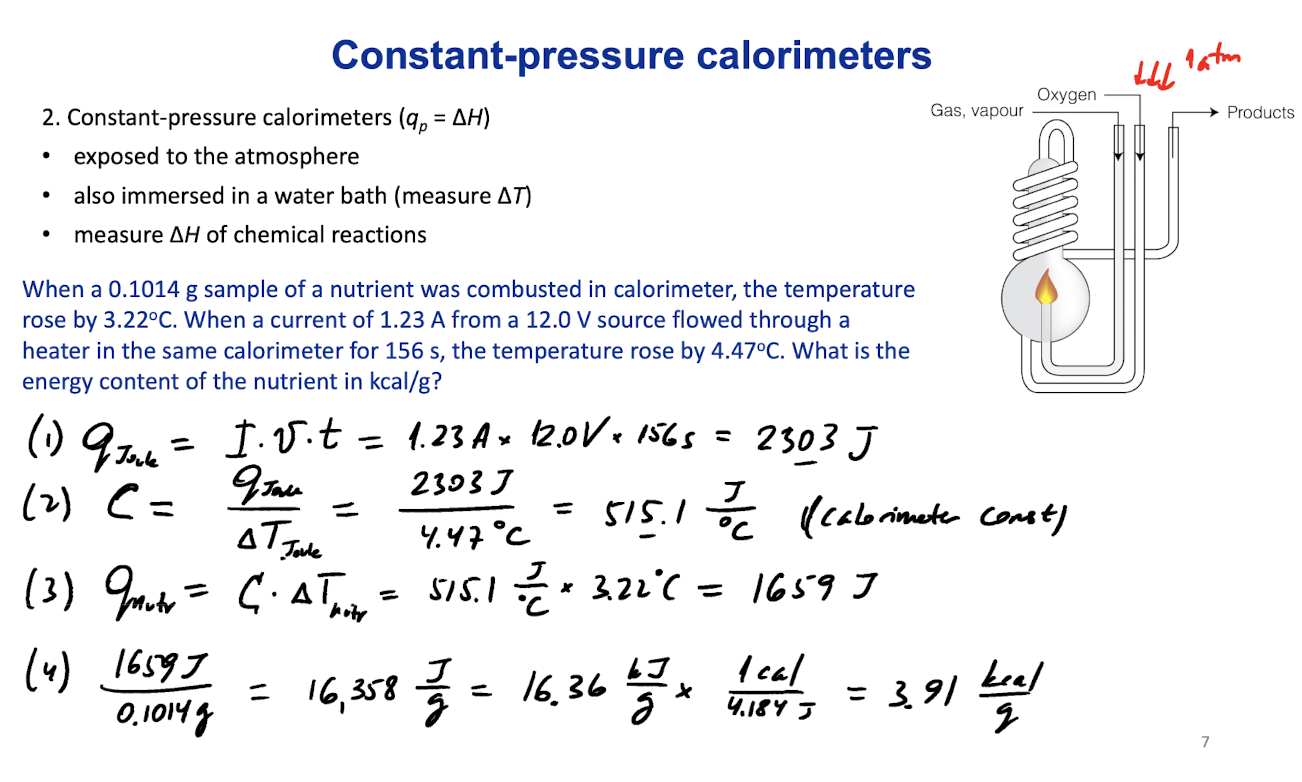
Constant pressure calorimeters
Constant-pressure calorimeters (qp=∆H)
exposed to the atmosphere
also immersed in a water bath (measure ∆T)
measure ∆H of chemical reaction
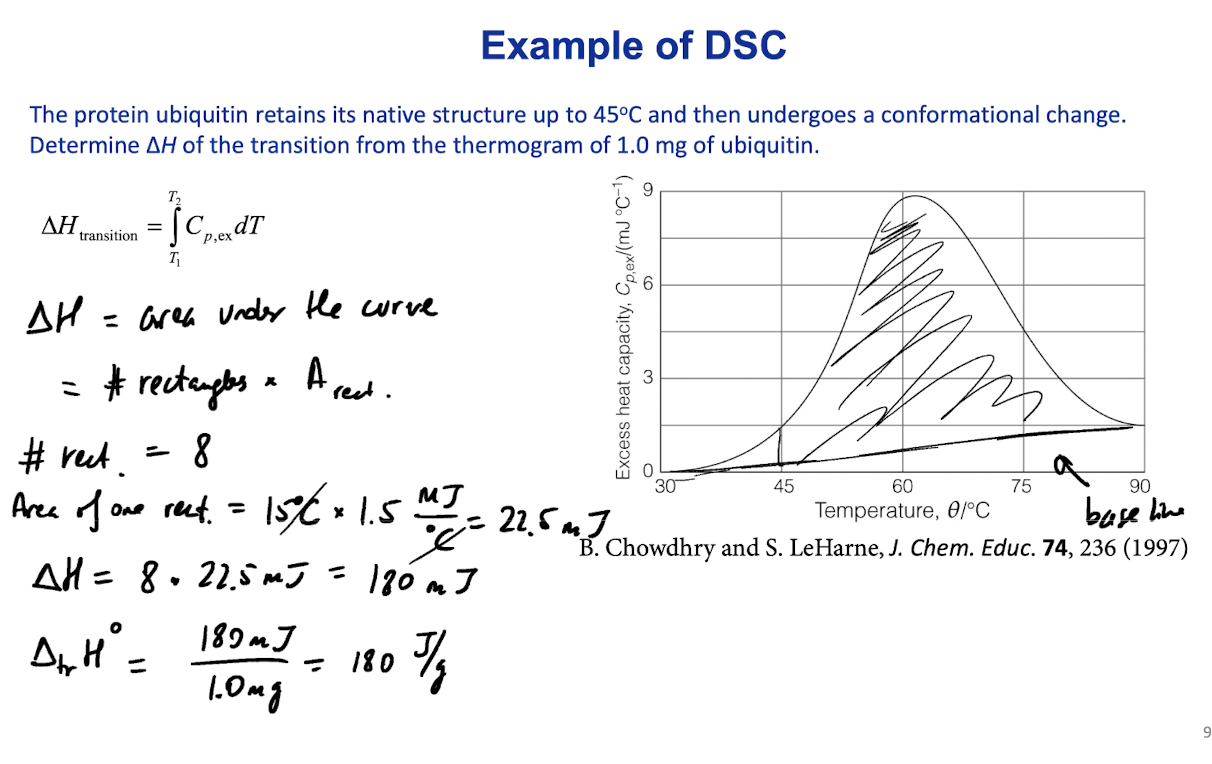
Differential scanning calorimeters
Differential scanning calorimeters (DSCs)
are used to measure ∆H of transitions
Heat a sample and a reference at a constant rate
The reference does not undergo through any transitions (e.g., boiling)
The sample and the ref. are heated uniformity
If the sample undergoes transition, it requires excess heat to maintain the same temperature as the sample:
qp,ex=qp,sample-qp,ref
Excess heat capacity: Cp,ex= (qp,ex/∆T)
∆H of a transition ∆Htransition=T1∫T2Cp,exdT