carboxylic acids and carboxylic acid derivatives
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
induction
having an electron withdrawing group. stabilizes a base.
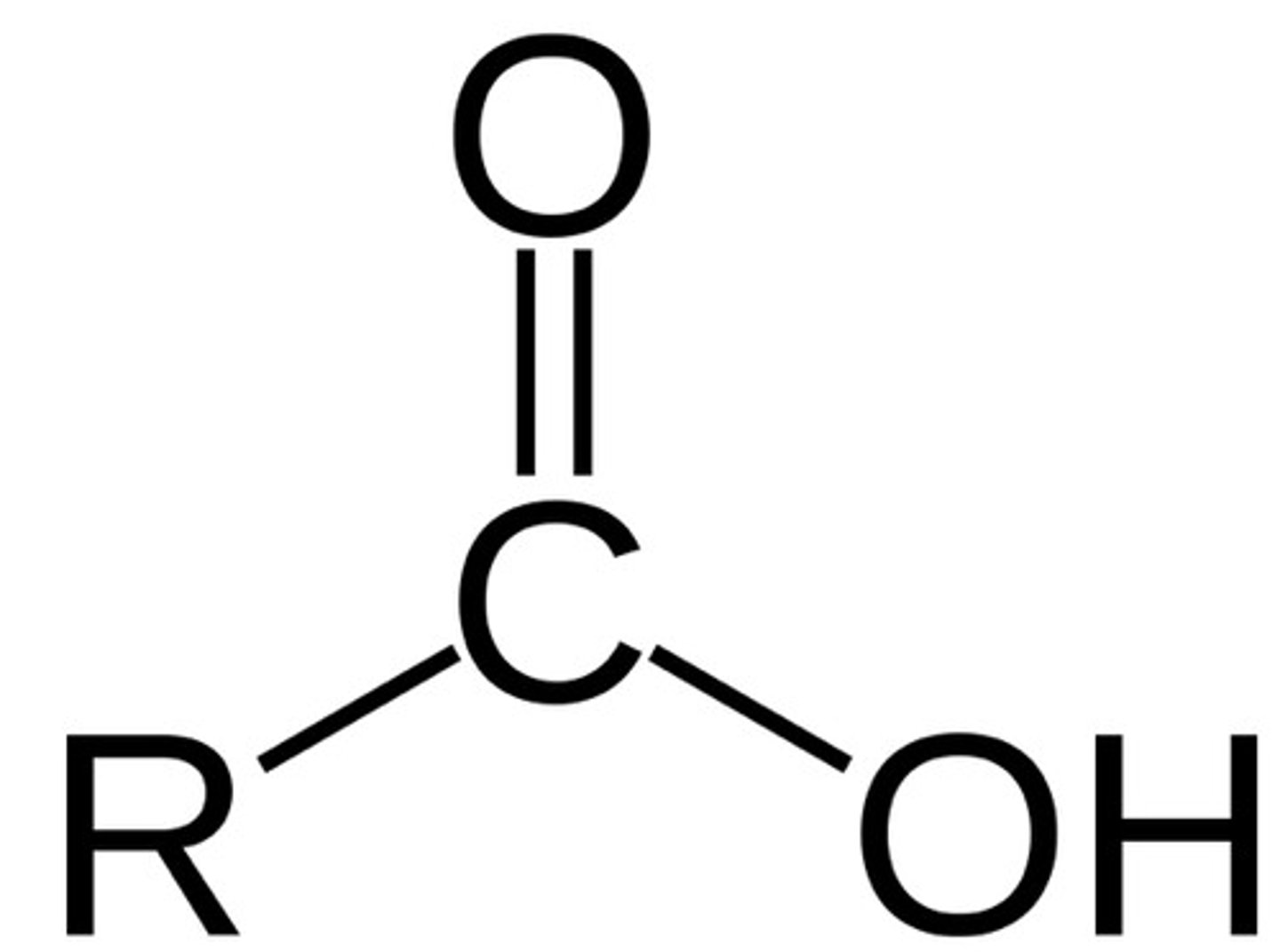
carboxylic acid
R-COOH. good electrophile due to dipole. always a terminal group
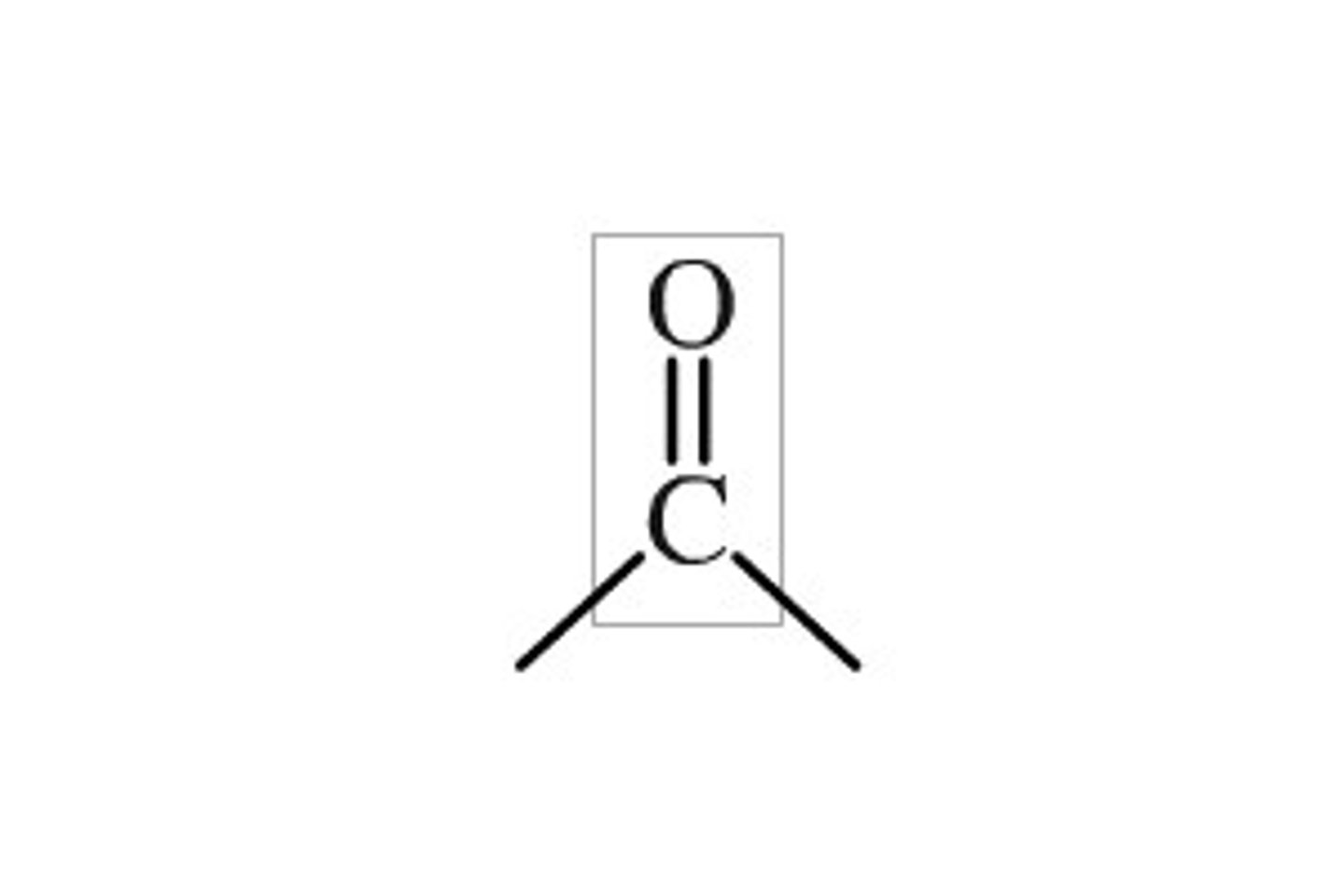
carbonyl
C=O
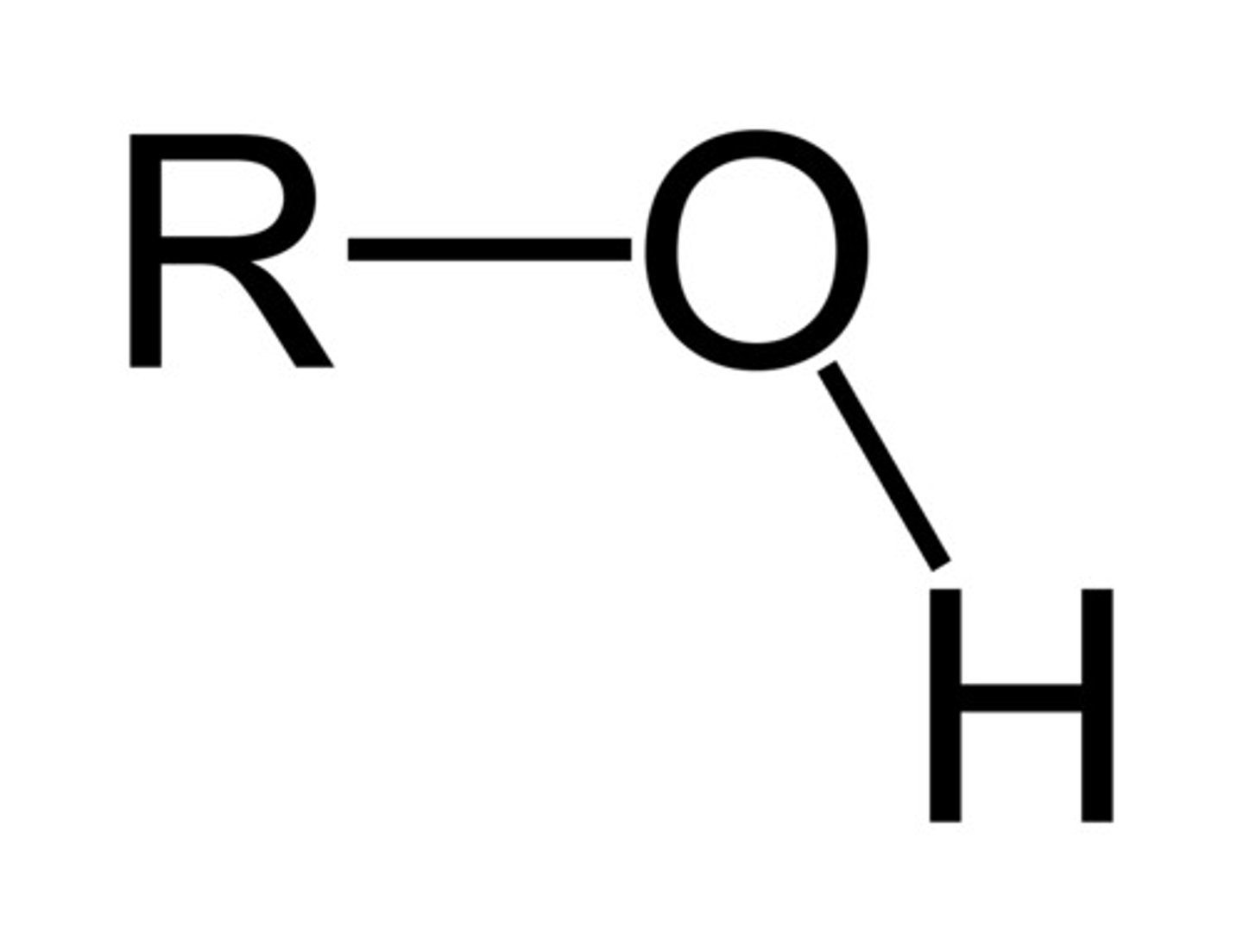
alcohol
R-OH
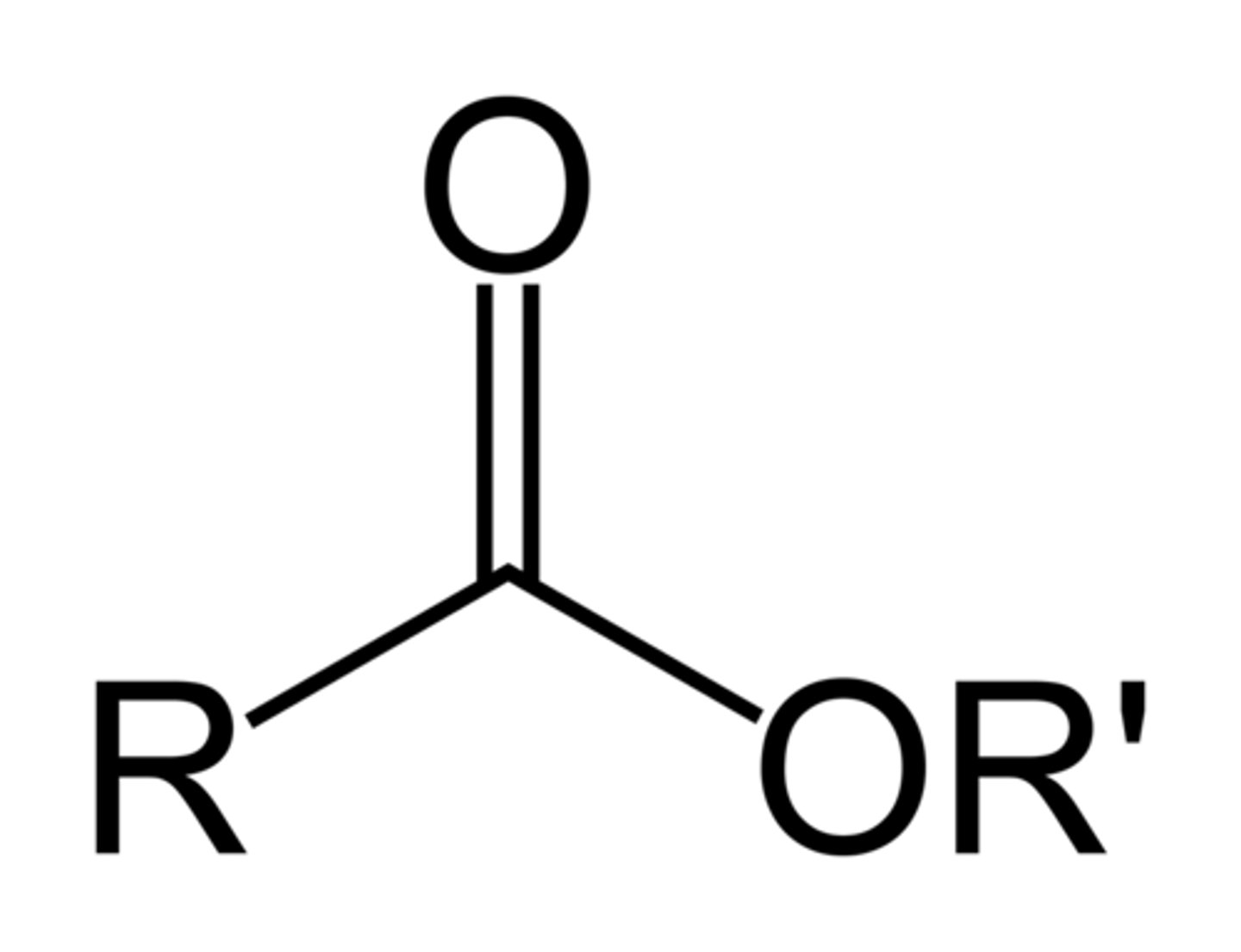
ester
RCOOR
typically found as the product of a condensation reaction with an alcohol and and acid.
nitrile
RCN
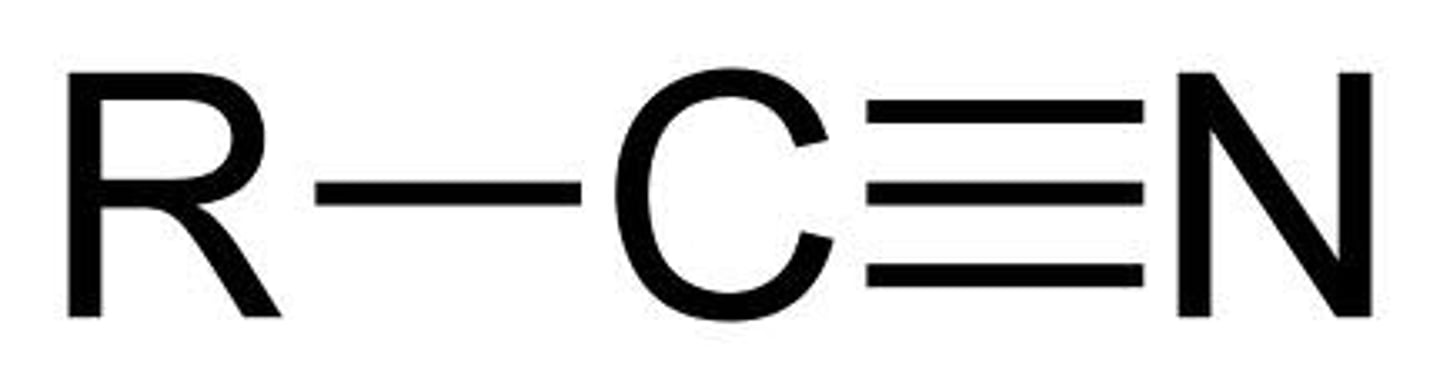
nucleophilic addition
addition reaction where a chemical compound with an electron-deficient or electrophilic double or triple bond (pi bond) reacts with electron rich reactant (nucleophile). double or triple bond disappears and creates two new single bonds
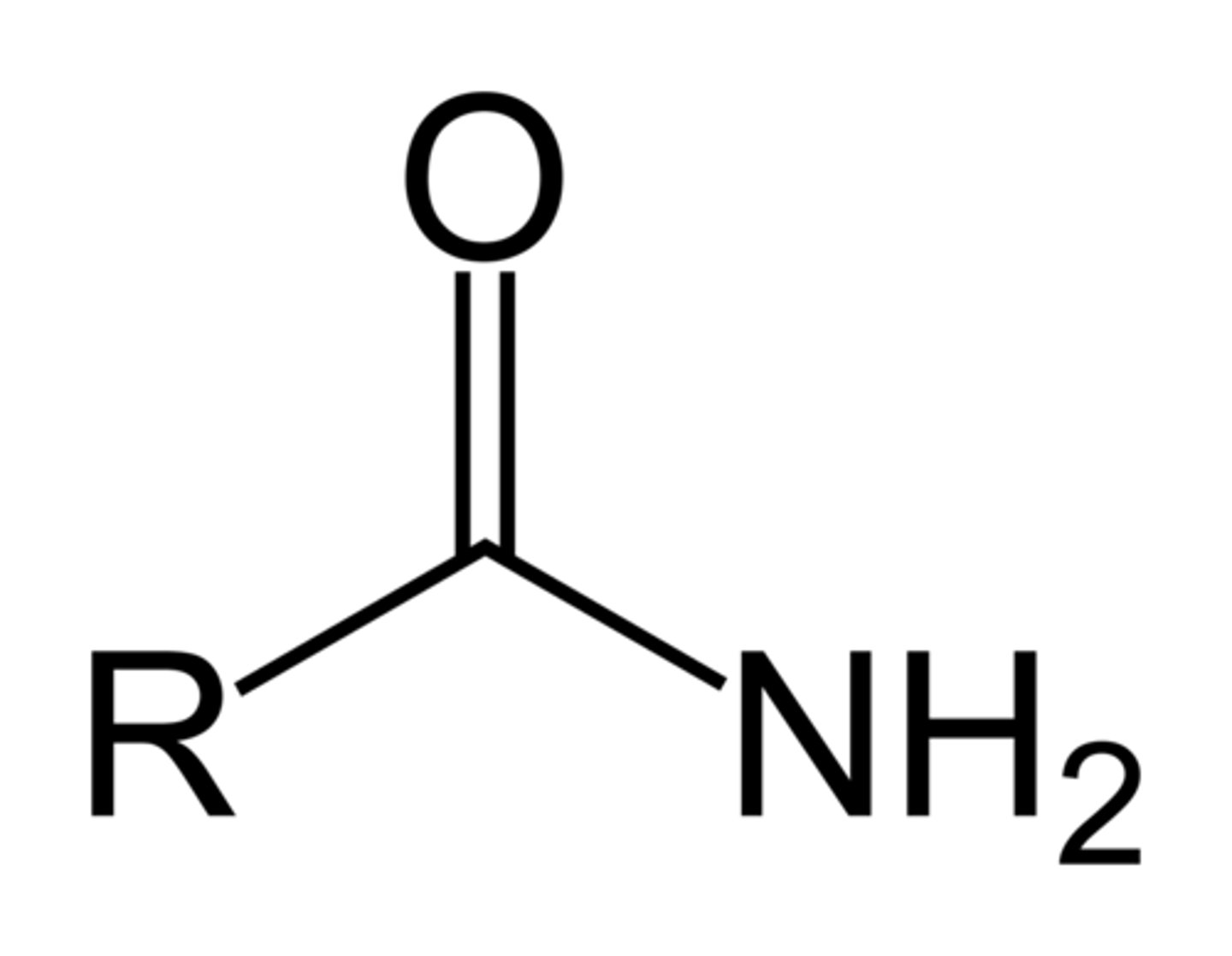
amide
NH2
fisher esterification
formation of ester from carboxylic acid and an alcohol initiated by an acid catalyst
acid catalyst protonates the alcohol --> forms oxonium ion --> protonates carboxylic acid --> carboxylic acid open to nucleophilic attack by the alcohol --> water eliminated yields protonated ester
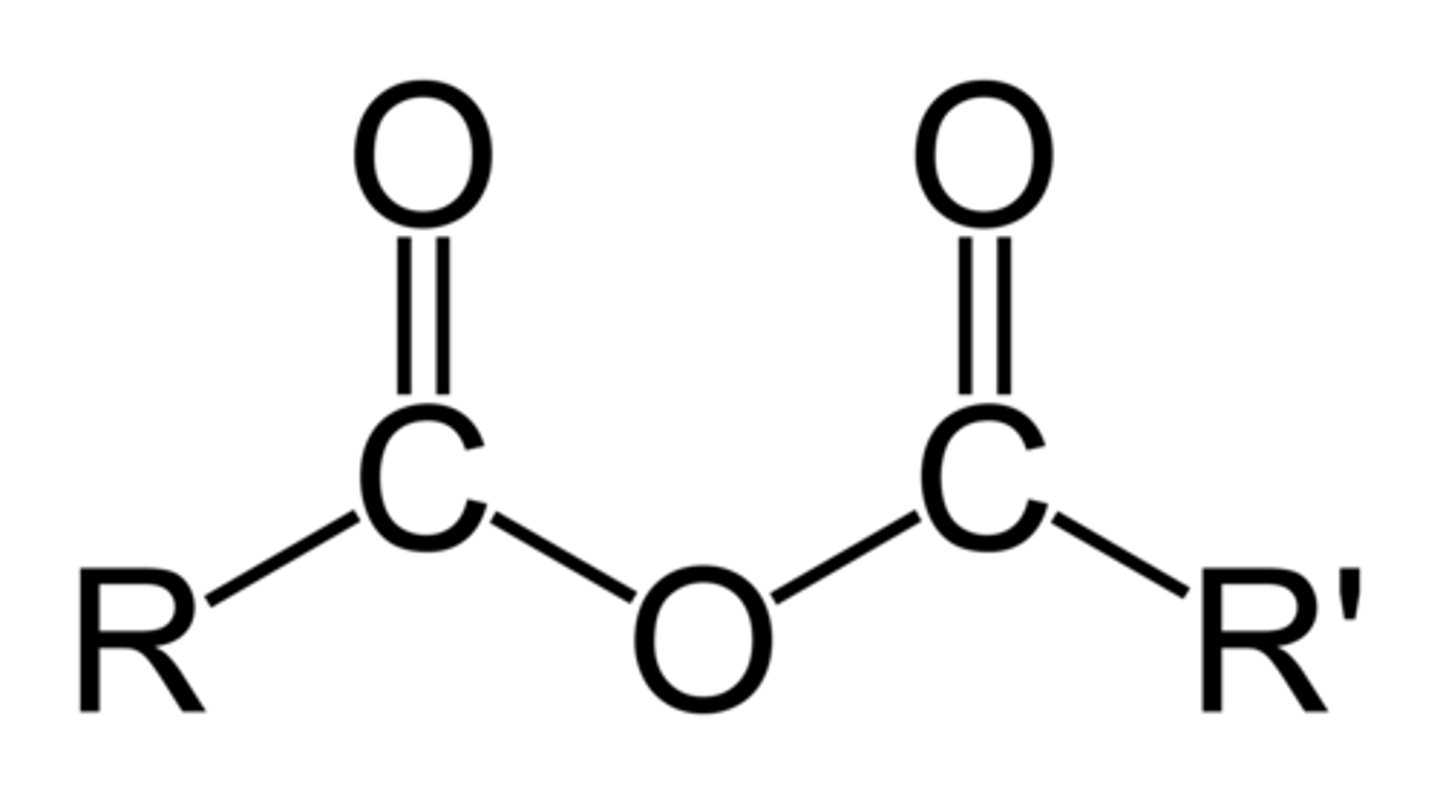
acid anhydride
an oxide that forms an acid when reacted with water
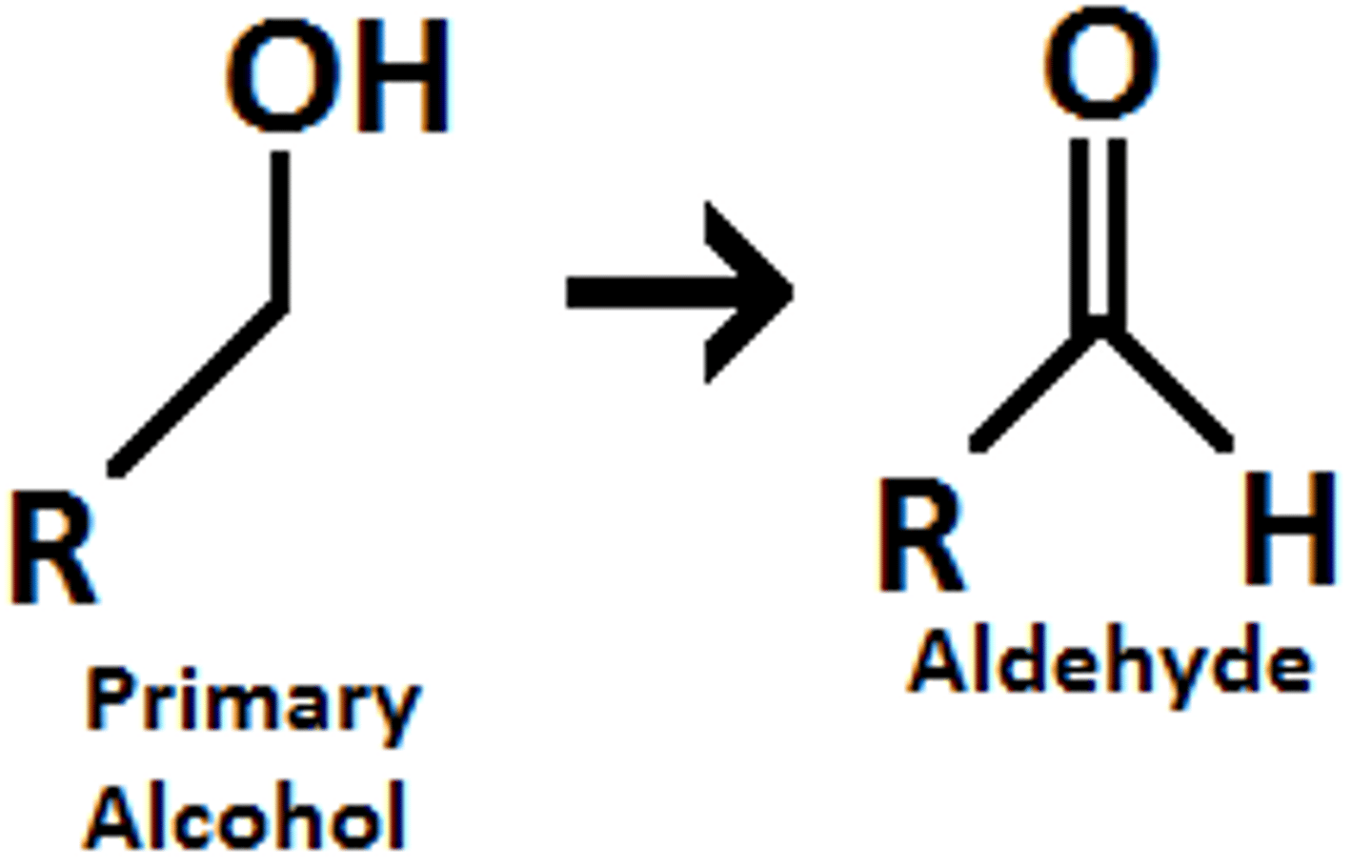
primary alcohols
only one carbon attached to the carbon with the hydroxyl functional group. can be oxidized to a ketone
decarboxylation
The complete loss of a carboxyl group as carbon dioxide
halogenation
The substitution of Hydrogen with one or more Halogens (Group VIIA elements).
nucleophile
An electron pair donor
electrophile
An electron pair acceptor
carboxylic acid nomenclature
-oic acid
carboxylate
deprotonated carboxylic acid. very stable. bases can deprotonate carboxylic acids
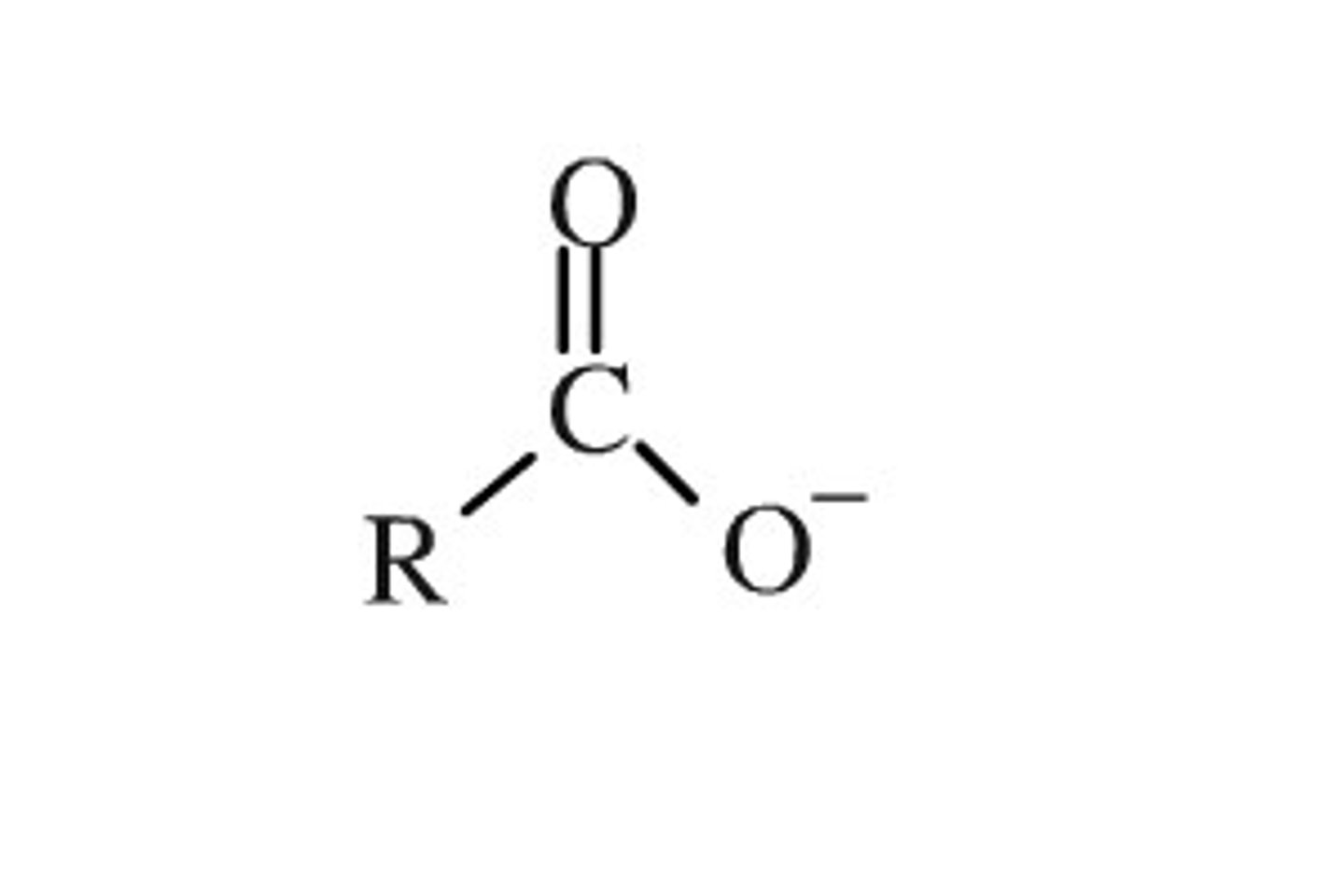
carboxylate nomenclature
-ate
acid proton is relatively acidic due to carboxylate stability. adding electron withdrawing groups to the the acid increases the acidity of the carboxylic acid. acidity increases with the number and proximity of the EWG
carboxylic acid acidity
nucleophilic acyl substitution
The substitution of a nucleophile for the leaving group of a carboxylic acid or carboxylic acid derivative.
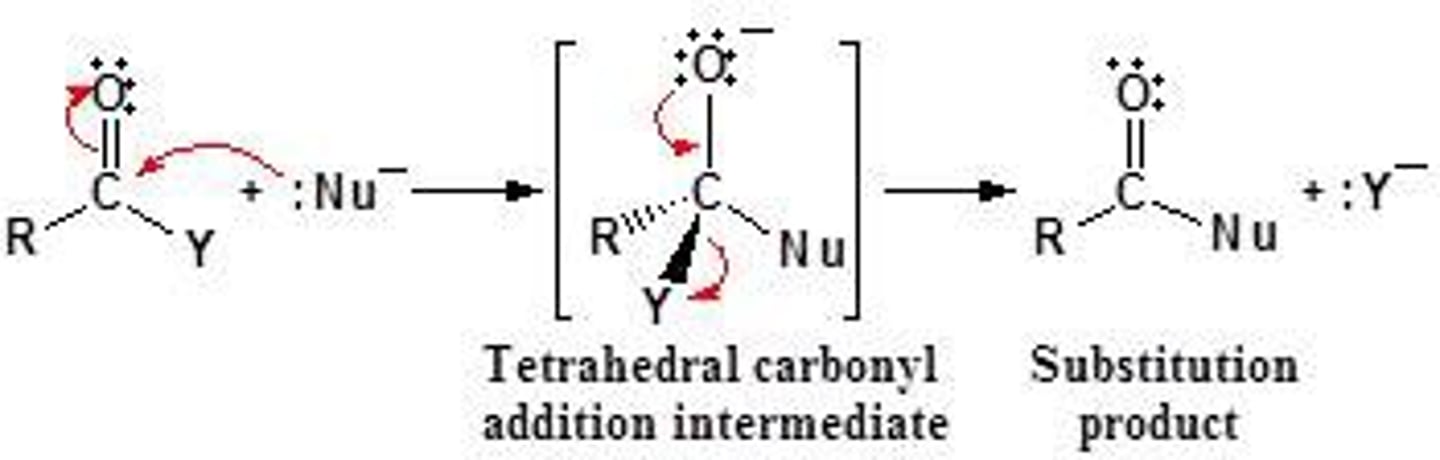
amide condensation
carboxylic acid to amide. amine (NH2-R) attacks carbonyl of carboxylic acid
esterification
carboxylic acid to ester. carboxyl is attacked by an alcohol. reaction is often catalyzed by an acid
ester nomenclature
-oate
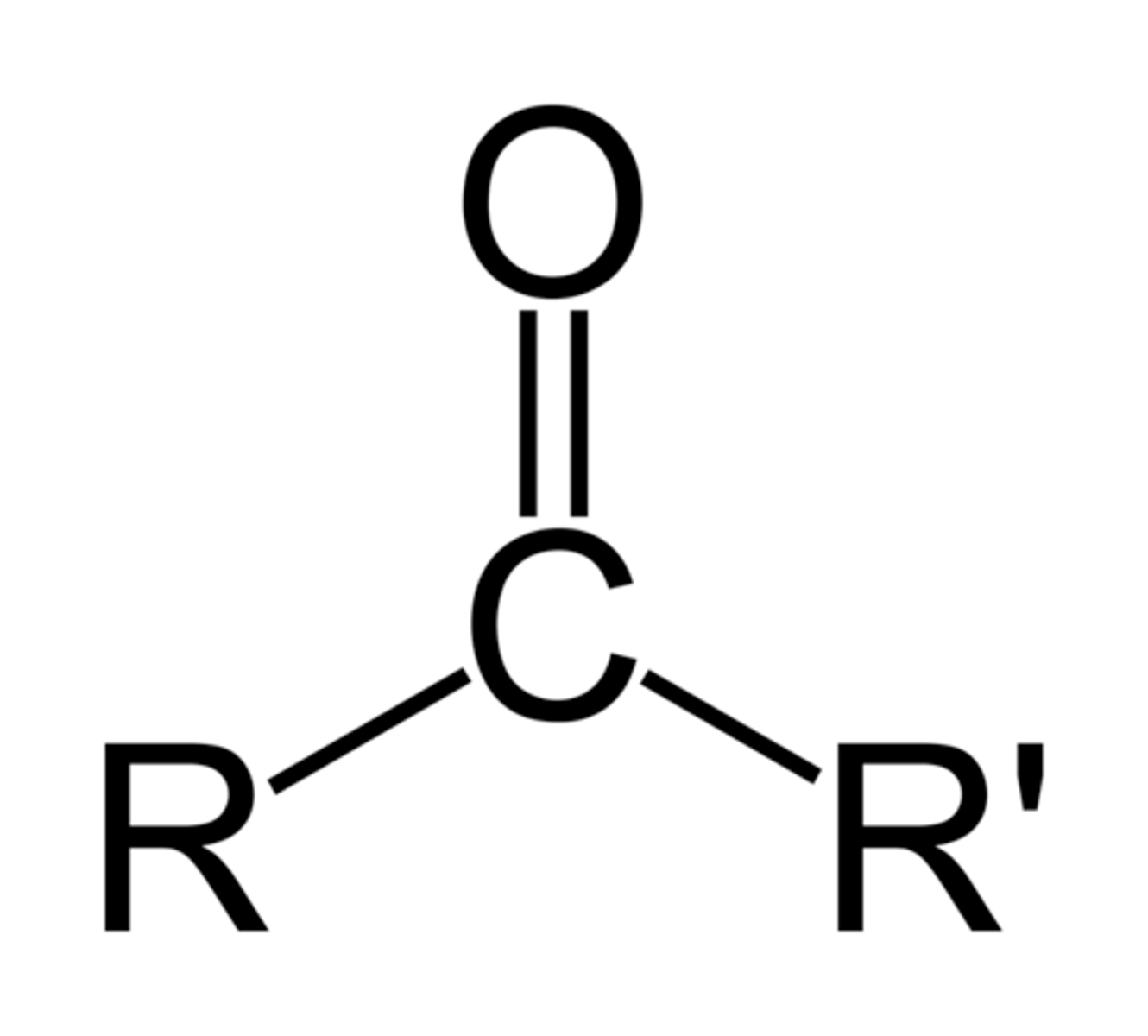
ketone
R-C=O-R. common electrophiles
oxidation
loss of electrons
reduction
gain of electrons
induces oxidation of primary alcohols and aldehydes to carboxylic acids, secondary alcohols to ketones
strong oxidizing agent reactions
Hot KMnO4
Na2Cr2O7
Jones reagent
H2CrO4
strong oxidizing agents
CrO3, H2SO4, and acetone
jones reagent
primary alcohol to aldehydes
secondary alcohols to ketone
weak oxidizing agent reactions
PCC
weak oxidizing agent. alcohols to aldehydes
PCC components
CrO3 and pyridine
carboxylic acids to primary alcohols
ketones to secondary alcohols
esters to alcohols
amides to amines
strong reducing agent reactions
LiAH4
strong reducing agent
aldehydes to primary alcohols
ketones to alcohols
weak reducing agent reactions
NaBH4
weak reducing agent
need oxidant
disulfide bond formation
need reductant. reactants like B-mercaptoethanol or dithiolthreiol (DTT) use their own sulfurs to create their own disulfide bonds
disulfide bond breaking
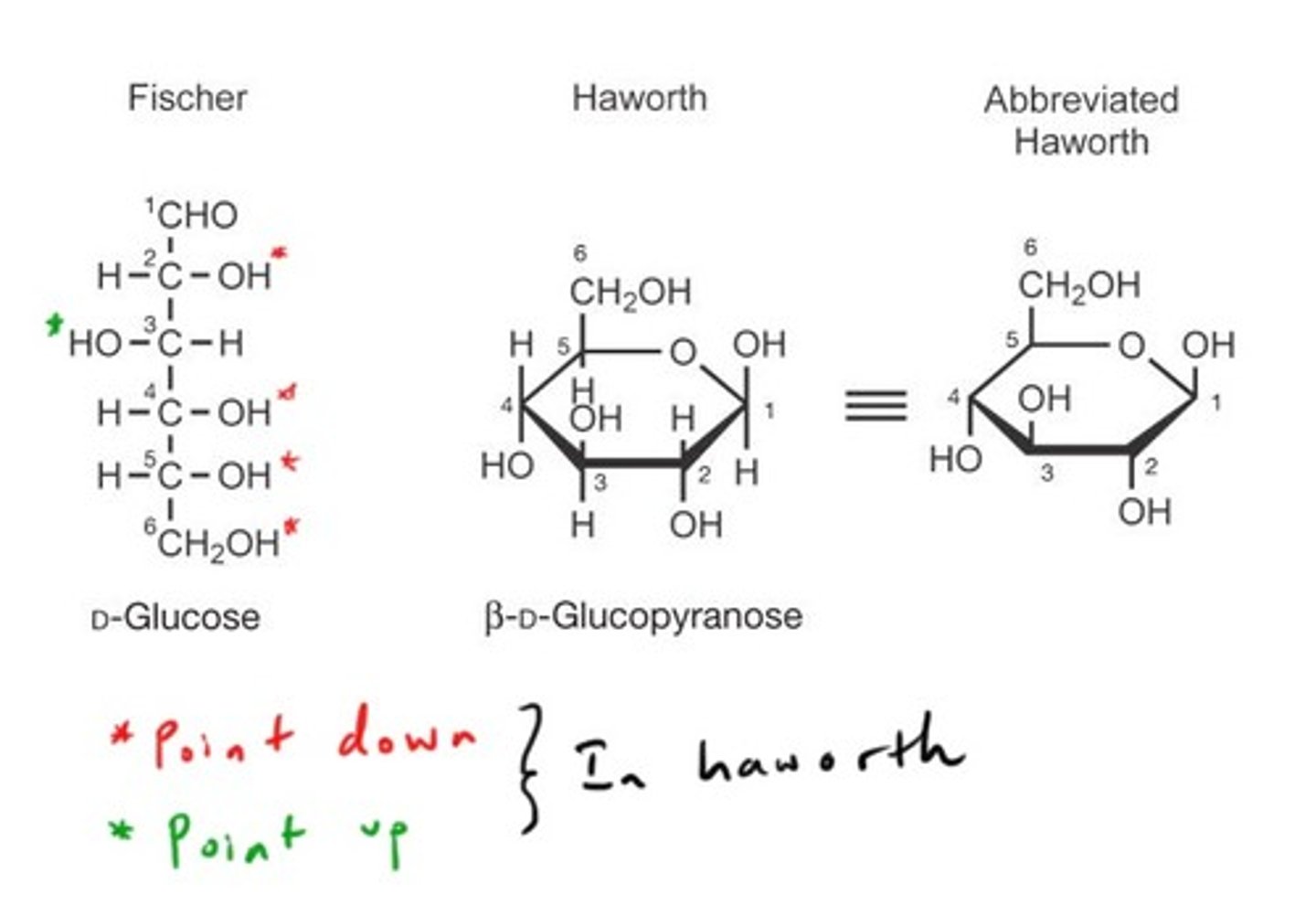
haworth projection
A method for depicting cyclic sugars as planar rings with -OH groups sticking up or down from the plane of the sugar
aldose
monosaccharide characterized by the presence of an aldehyde functional group
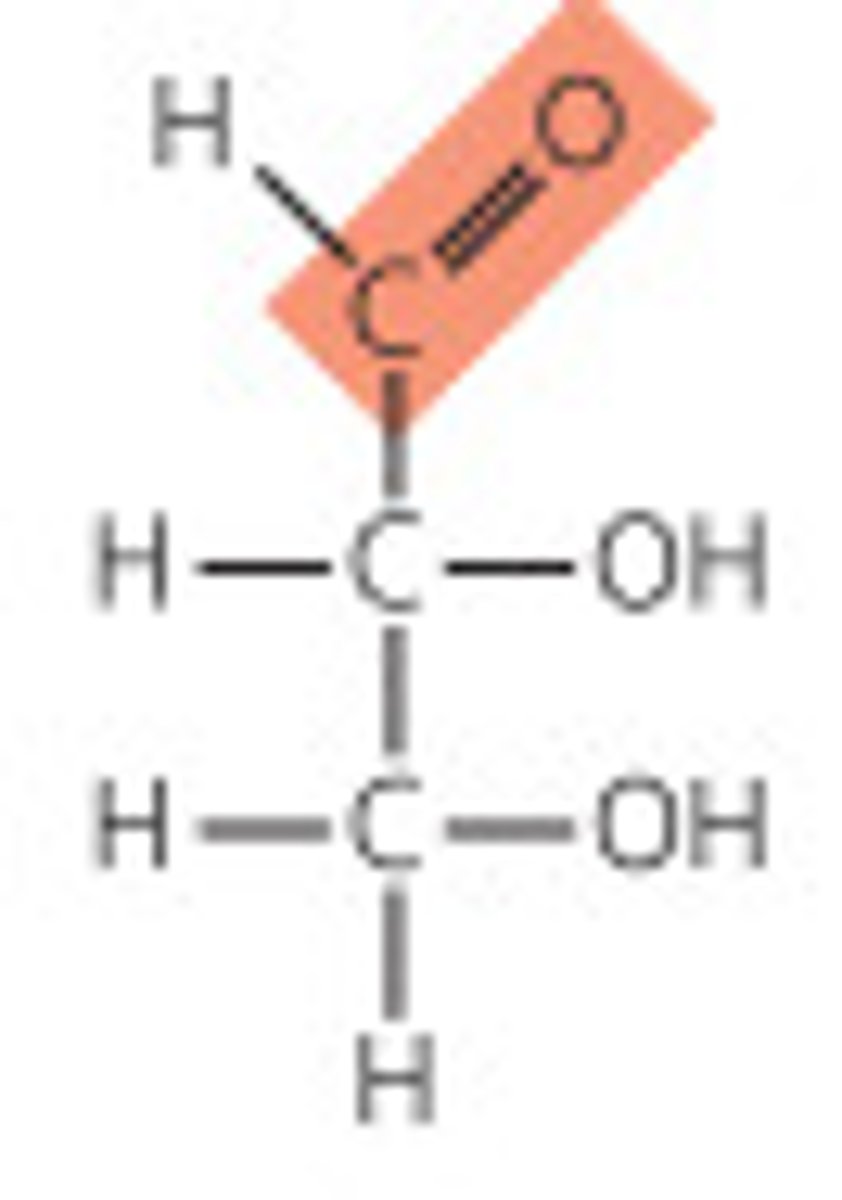
ketose
monosaccharide characterized by the presence of a ketone
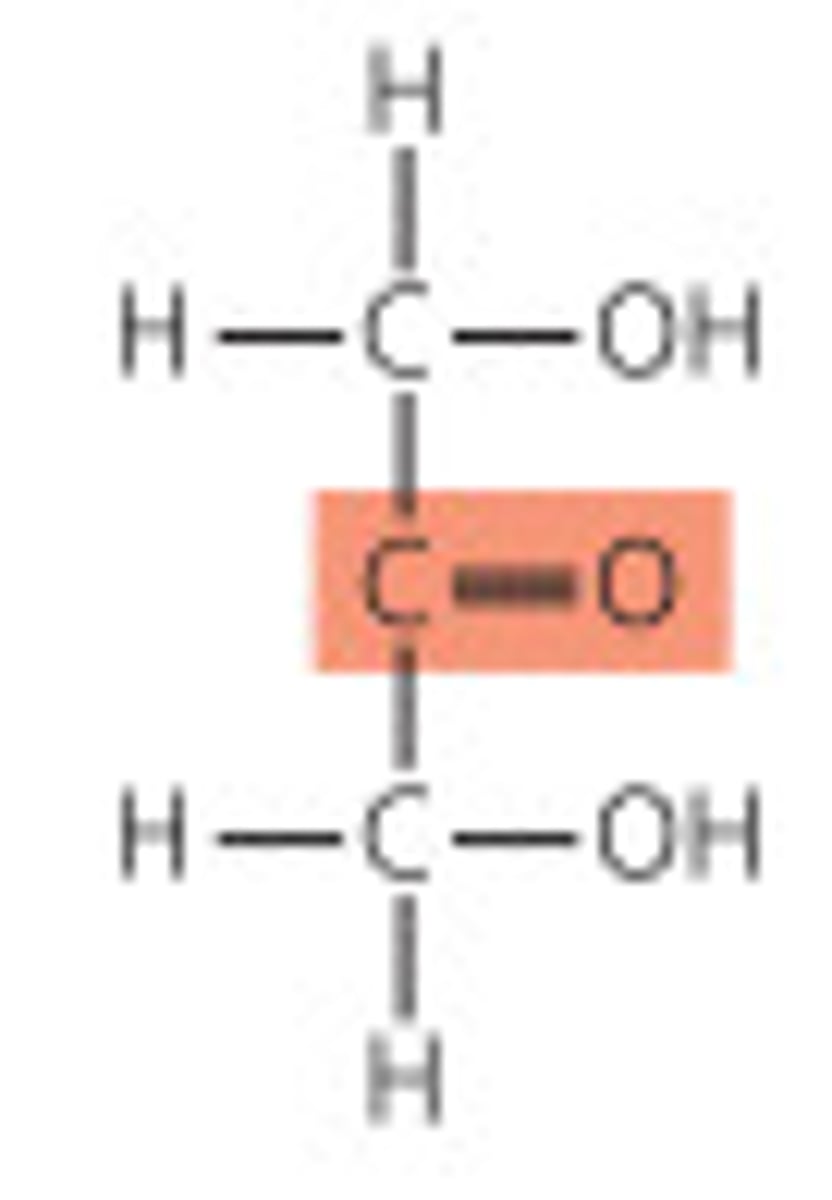
cyclization of sugars
aldehyde to hemiacetal or ketone to hemiketal. anomeric carbon is a new chiral center
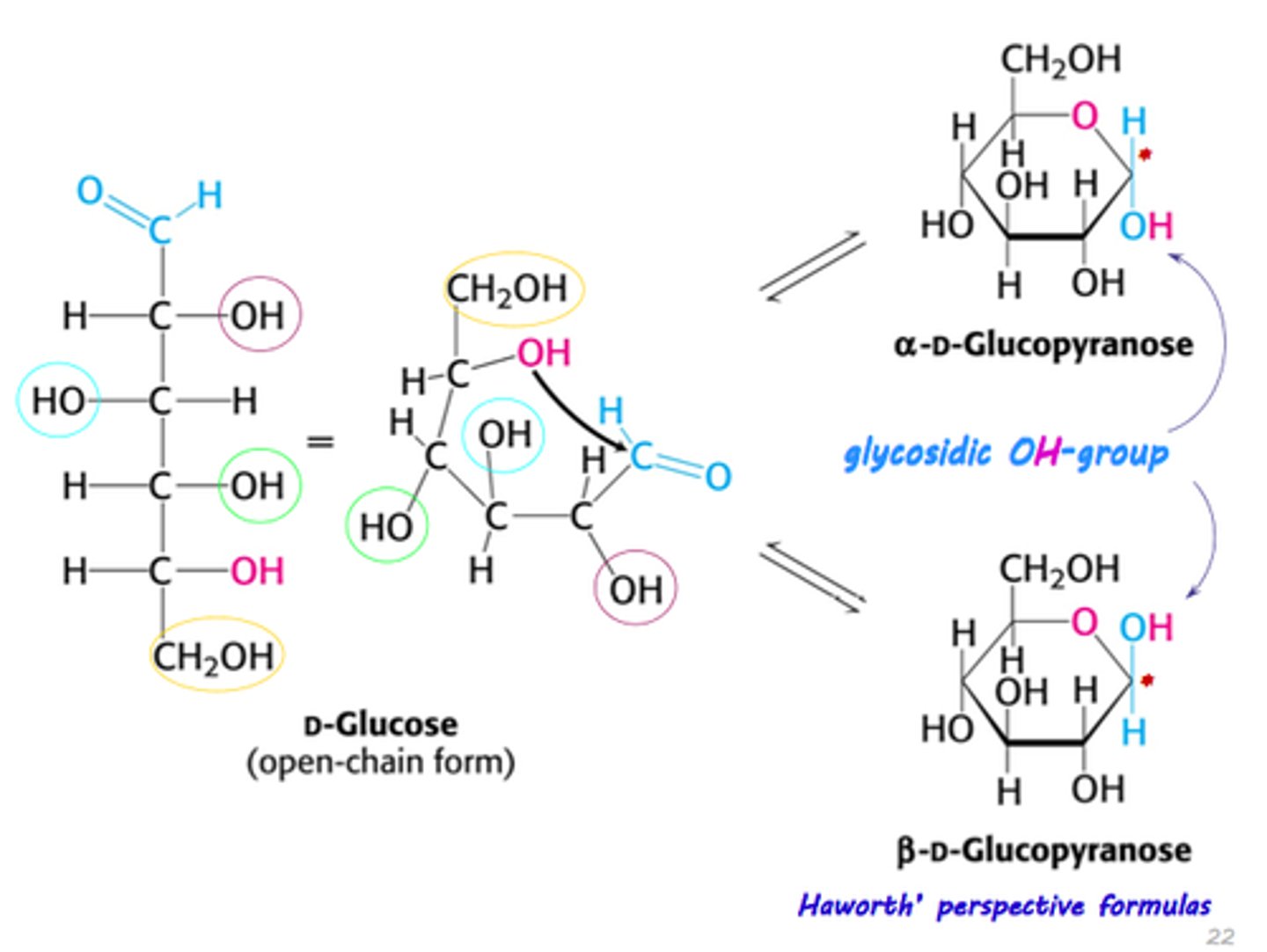
hemiacetal in sugars
when the O on carbon 5 attack the C1 carbon to create a ring. the C1 carbon becomes the anomeric carbon
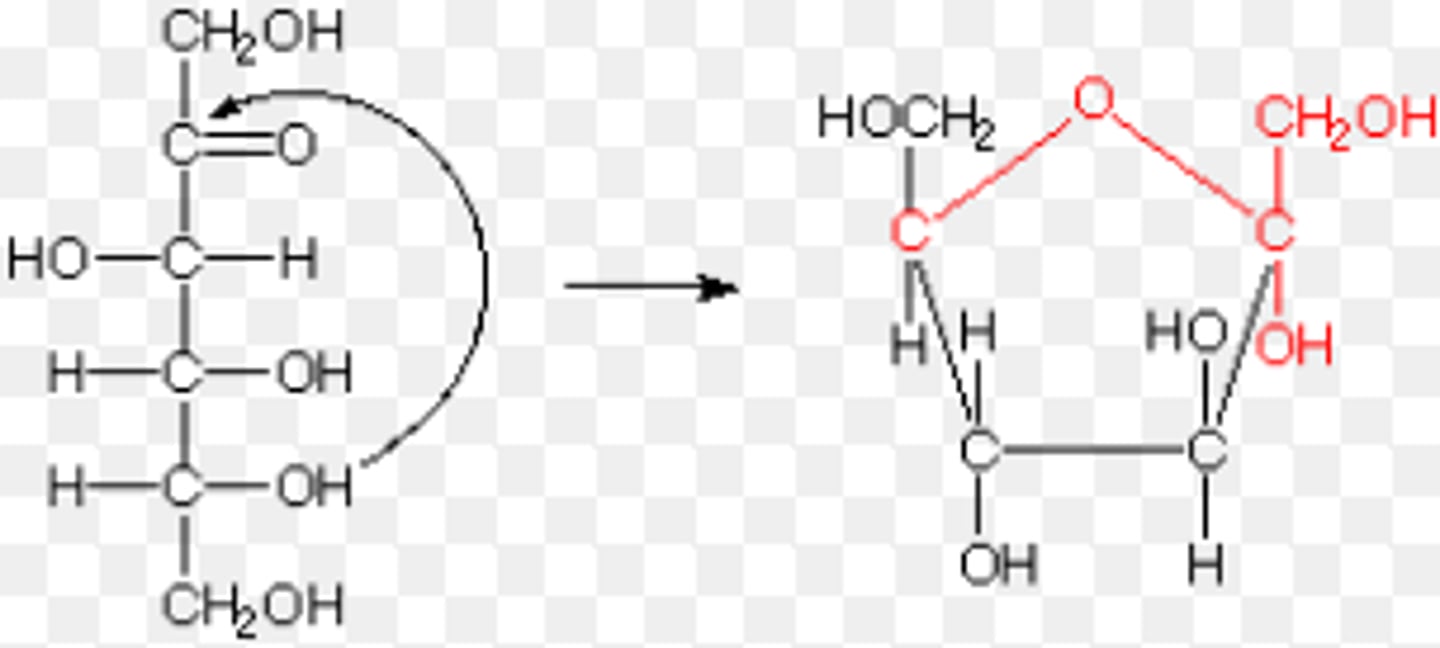
hemiketal in sugars
when the O on carbon 5 attacks the C2 carbon to create a ring. the C2 carbon becomes the anomeric carbon
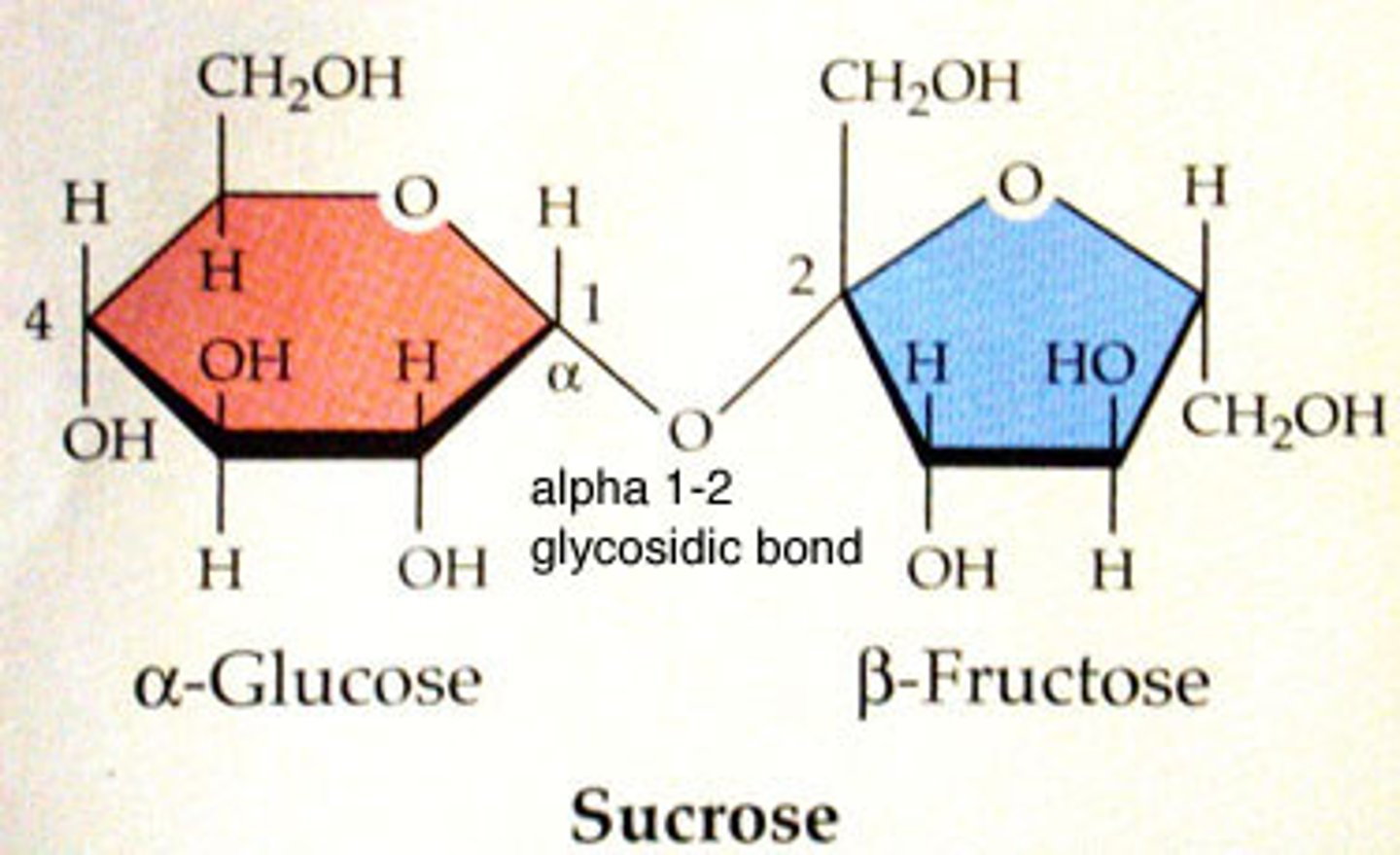
sucrose
won't yield a positive on Tollen's test
mutarotation
The rapid interconversion between different anomers of a sugar
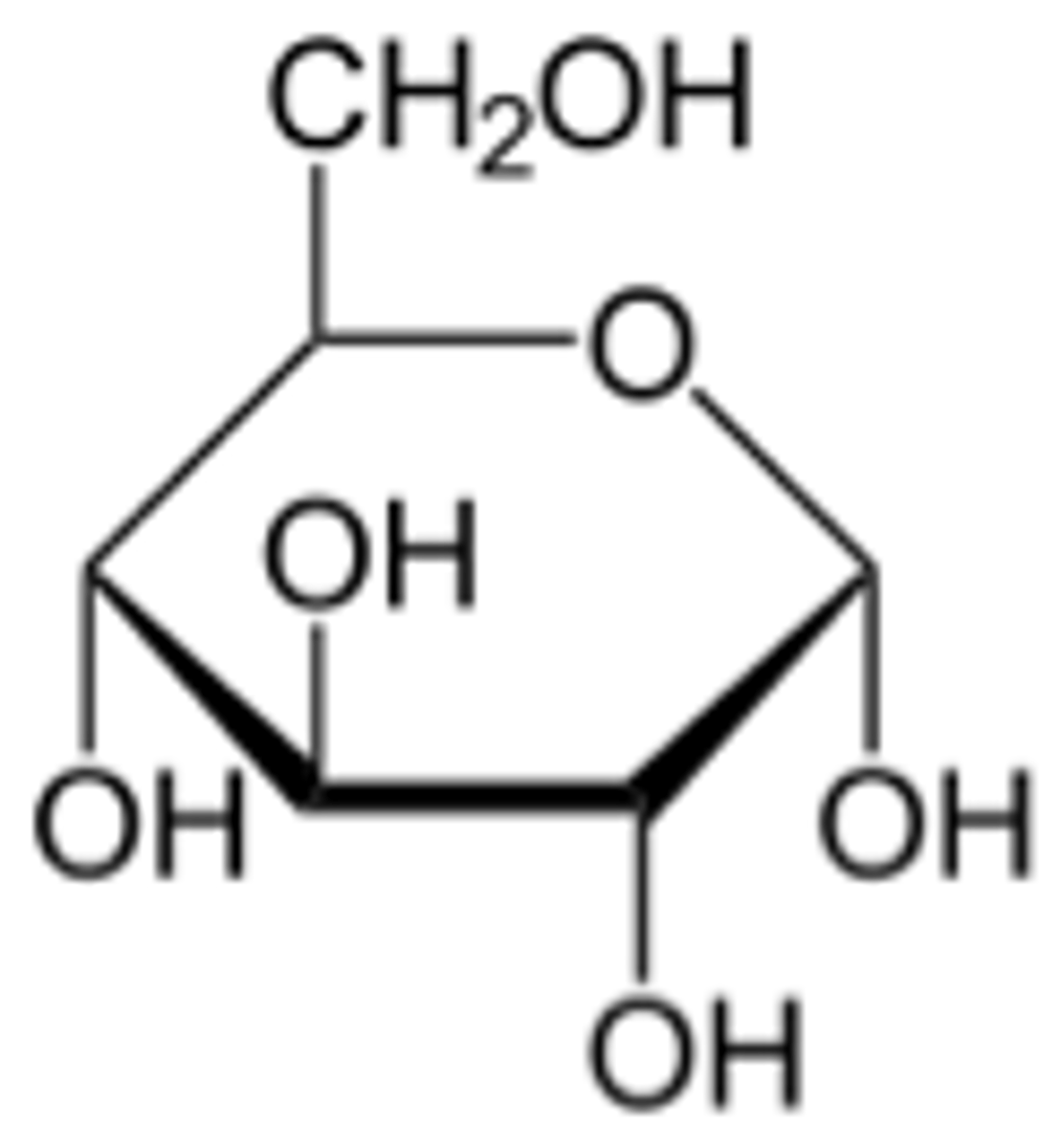
beta-anomer
the OH group of the C1 cis to the CH2OH group
it will be equatorial and up
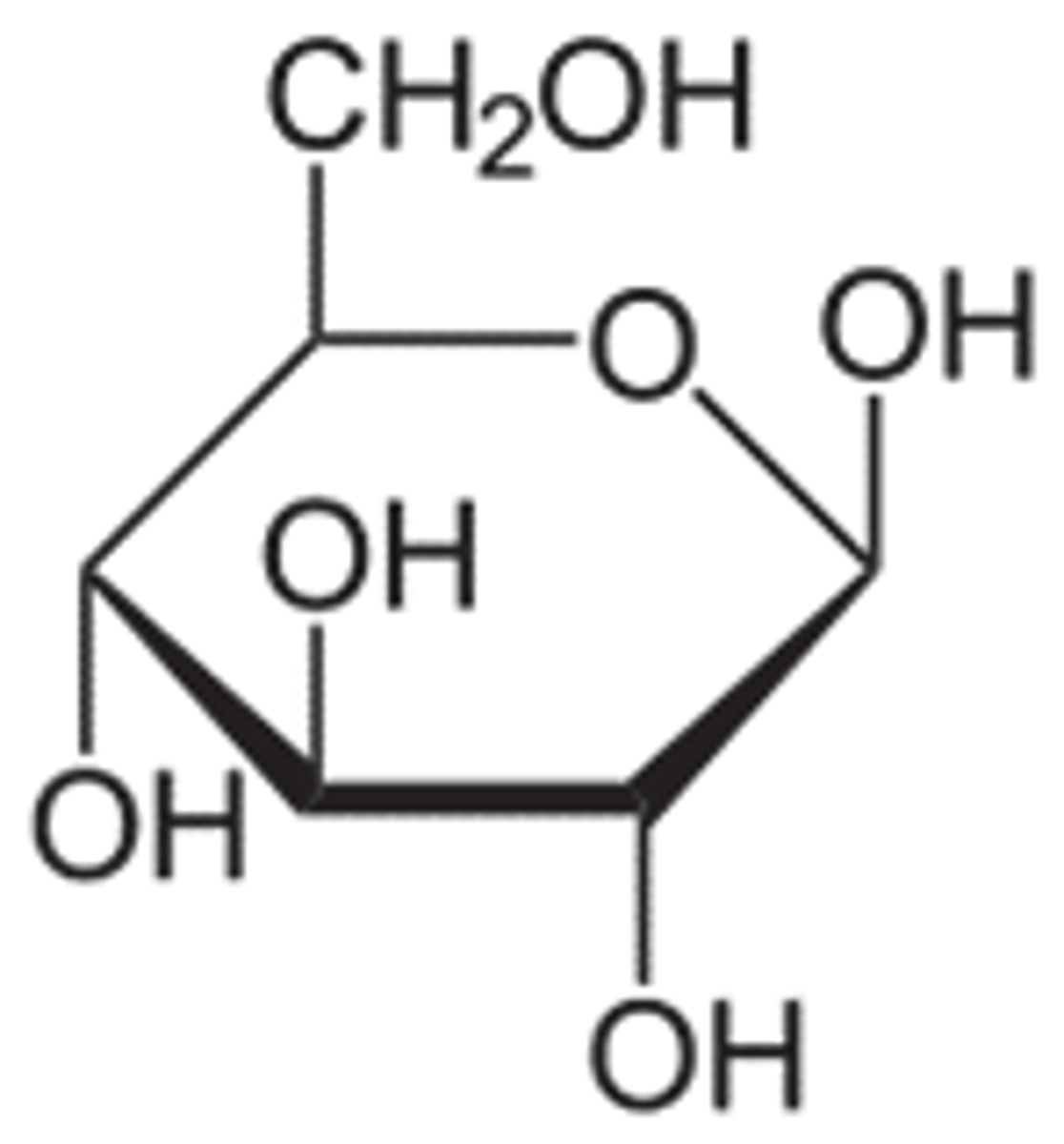
alpha-anomer
OH group in the C1 is trans to the CH2OH
it will be axial and down
tollen's reagent
uses silver nitrate as an oxidizing agent;
positive test results in aldehydes reducing Ag+ to metallic silver
Benedict's solution
A chemical indicator that, when added to a solution and heated, changes from blue to light green to red in the presence of increasing concentrations of sugar.
fehling's test
Aldehyde can reduce Cu2+ to Cu+
reducing sugars
Sugars that produce a red precipitate when boiled with Benedict's solution. aldoses or terminal alpha-hydroxy ketones. E.g. glucose, maltose, fructose, lactose.
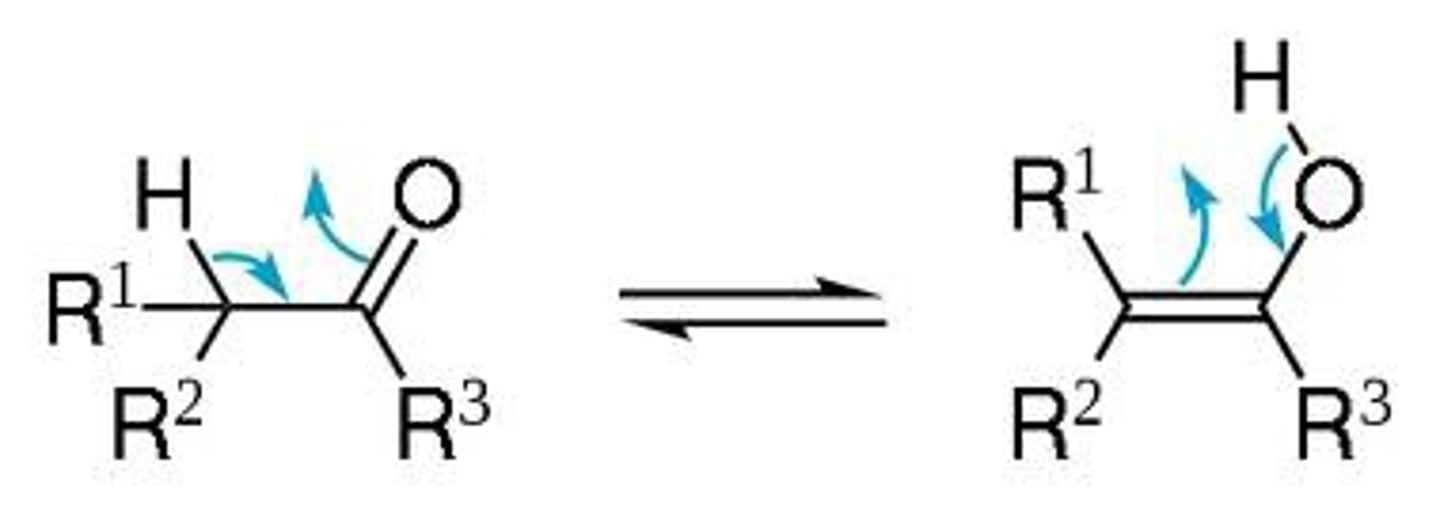
tautomerization
the rearrangement of bonds in a compound, usually by moving a hydrogen and forming a double bond
partial positive or positive charge makes a good
electrophile
partial negative or full negative charge makes a good
nucleophile
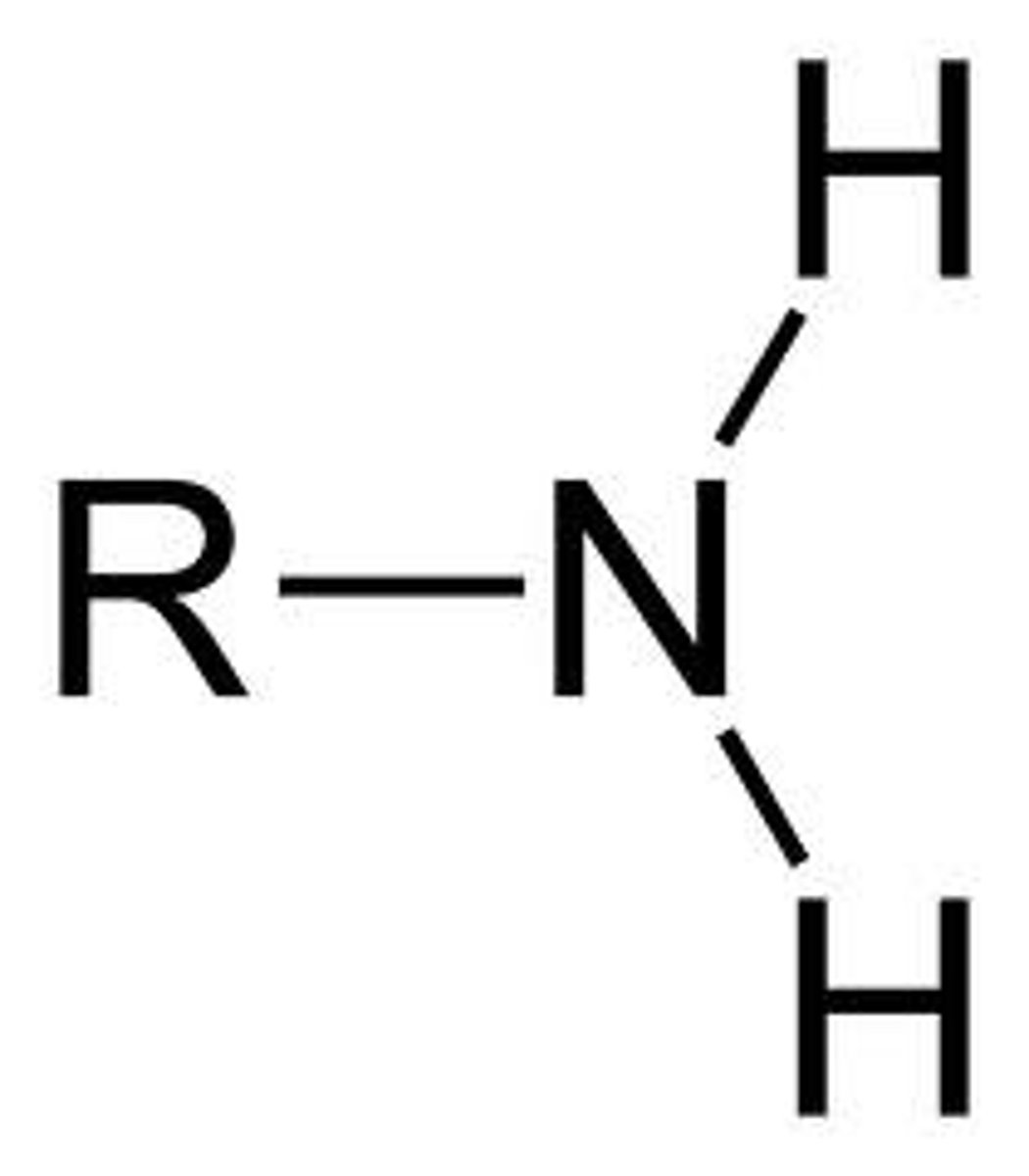
amine
NH2
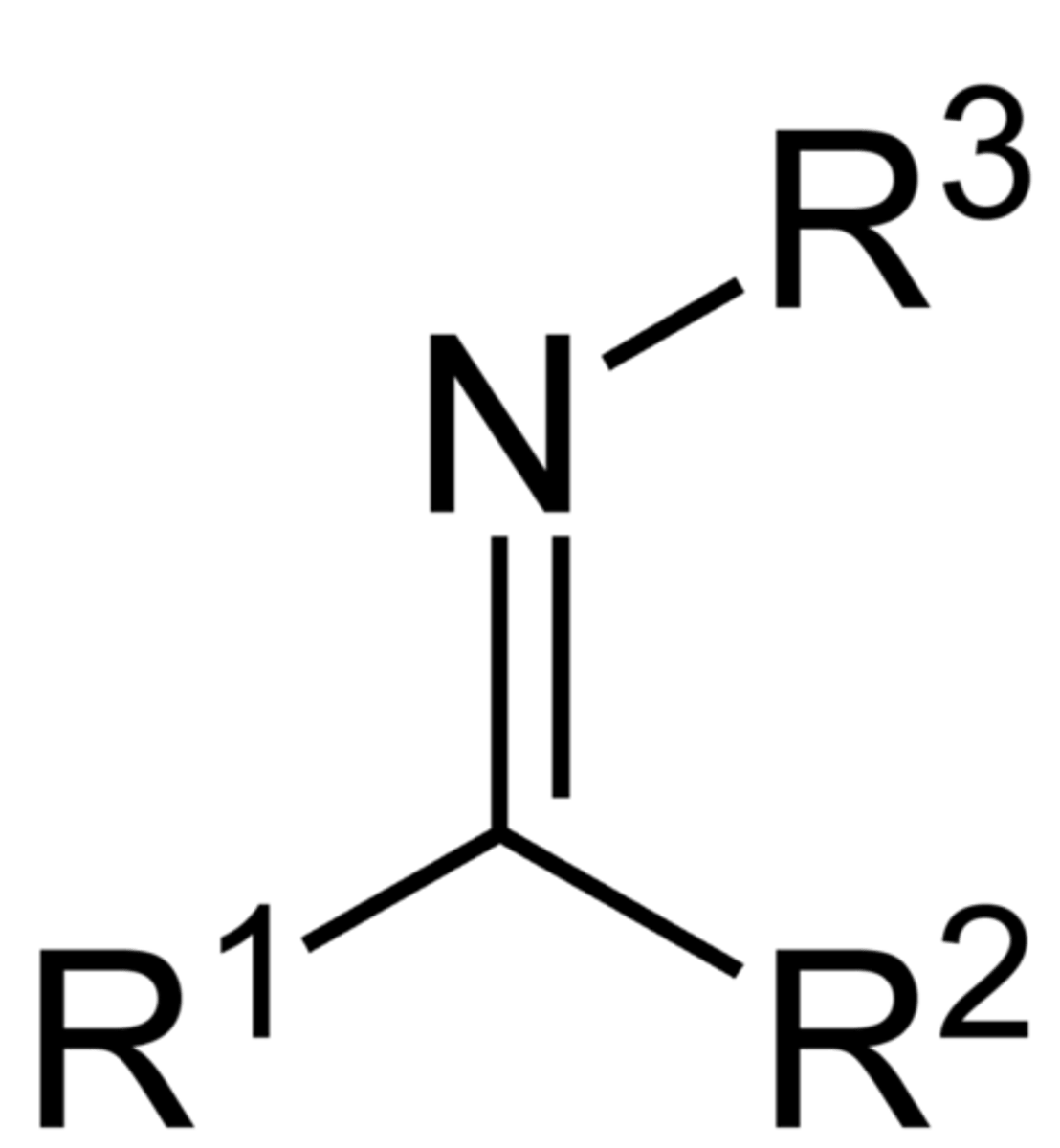
imine
A double bond between a carbon and a nitrogen
SN1 reaction
*unimolecular nucleophilic substitution reactions: 2 steps
1. Leaving group leaves forming a positively charged carbocation (rate limiting step)
*The rate of rxn depends only on the concentration of the substrate
rate = k [R-L]
R-L is the alkyl group containing the leaving group
*Anything that accelerates the formation of the carbocation increase the rate of rxn
2. Nucleophile attacks the carbocation (unstable)
*results in substitution product
![<p>*unimolecular nucleophilic substitution reactions: 2 steps</p><p>1. Leaving group leaves forming a positively charged carbocation (rate limiting step)</p><p>*The rate of rxn depends only on the concentration of the substrate</p><p>rate = k [R-L]</p><p>R-L is the alkyl group containing the leaving group</p><p>*Anything that accelerates the formation of the carbocation increase the rate of rxn</p><p>2. Nucleophile attacks the carbocation (unstable)</p><p>*results in substitution product</p>](https://knowt-user-attachments.s3.amazonaws.com/69ad3e4f-d852-45e5-8ff7-1a8c5e413925.jpg)
SN2 reaction
-bimolecular nucleophilic substitution reactions
- only 1 step (concerted reaction)
-nucleophile attacks the compound at the same time as the leaving group leaves
-Nucleophile actively displaces the leaving group in a backside attack
for this to occur, nucleophile must be strong & substrate can't be sterically hindered
-concentrations of substrate & nucleophile have role in determining the rate --> rate = k[Nu][R-L]
-Position of the substituents around the substrate carbon is inverted
![<p>-bimolecular nucleophilic substitution reactions</p><p>- only 1 step (concerted reaction)</p><p>-nucleophile attacks the compound at the same time as the leaving group leaves</p><p>-Nucleophile actively displaces the leaving group in a backside attack</p><p>for this to occur, nucleophile must be strong & substrate can't be sterically hindered</p><p>-concentrations of substrate & nucleophile have role in determining the rate --> rate = k[Nu][R-L]</p><p>-Position of the substituents around the substrate carbon is inverted</p>](https://knowt-user-attachments.s3.amazonaws.com/a376f404-73eb-4b62-a7c4-56126fa5da85.png)
acetyl group
COCH3
Biologically important carboxylic acids include pyruvic acid, citric acid, and the C-terminus of amino acid chains. Which of the following carboxylic acids will be the most acidic?
A. CH3CHClCH2COOH
B. CH3CH2CCl2COOH
C. CH3CH2CHClCOOH
D. CH3CH2CH2COOH
B.
carboxylic acids and acidity
carboxylic acids and their derivatives increase in acidity as the number of electron withdrawing groups increase. the closer in proximity to the acid functionality is to the electronegative group
is OH a better nucleophile or electrophile
nucleophile
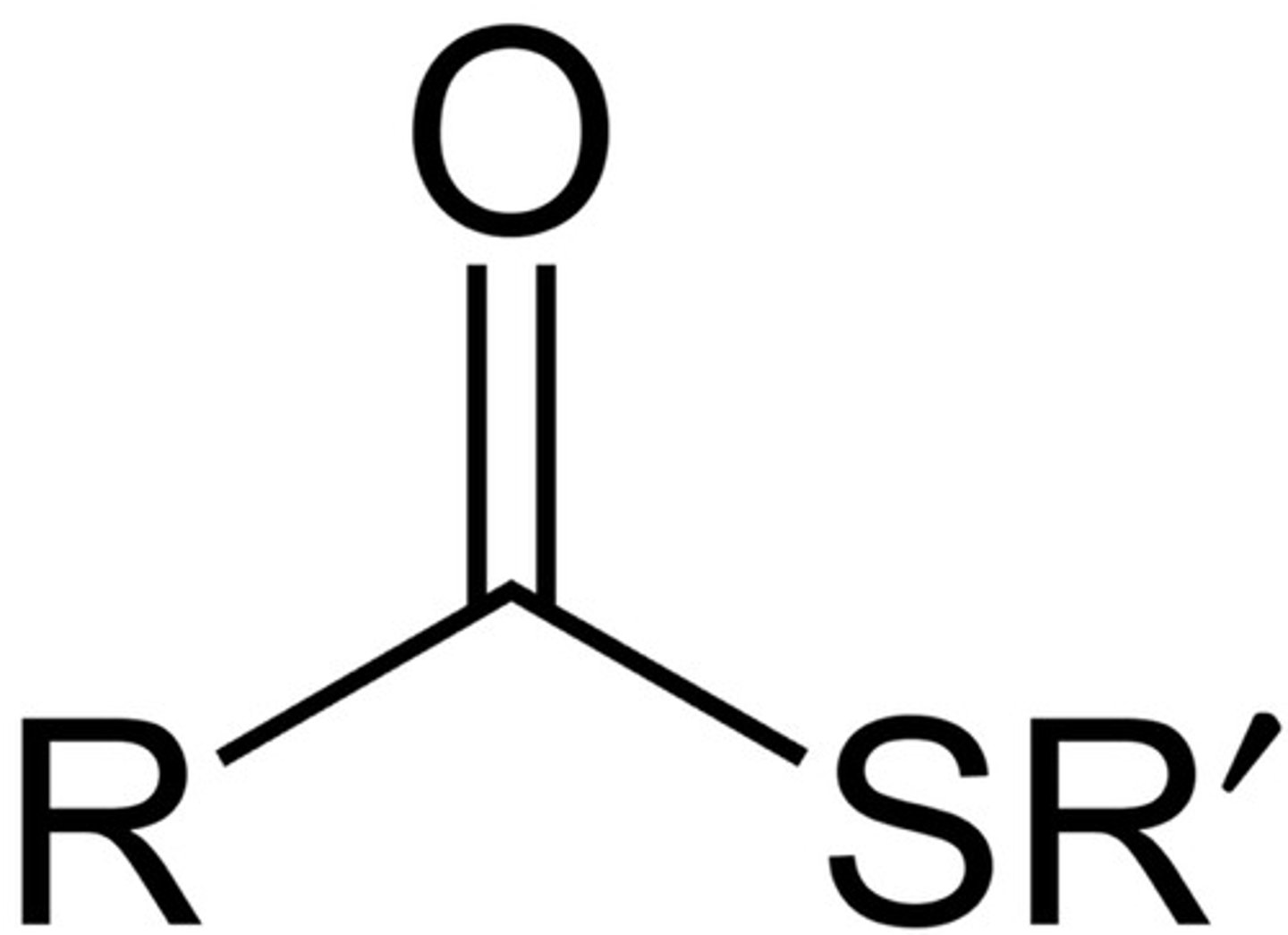
thioester
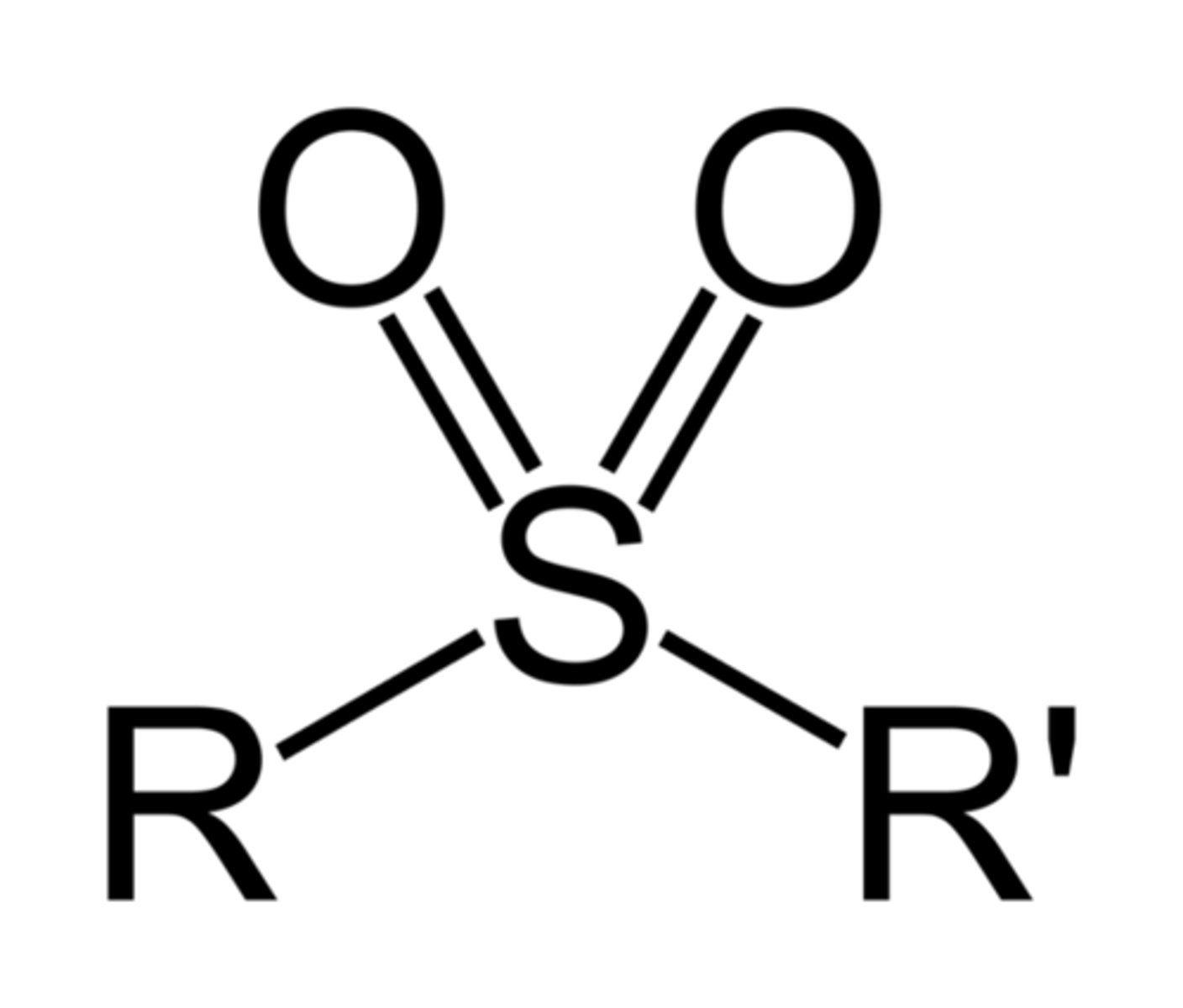
sulfonyl
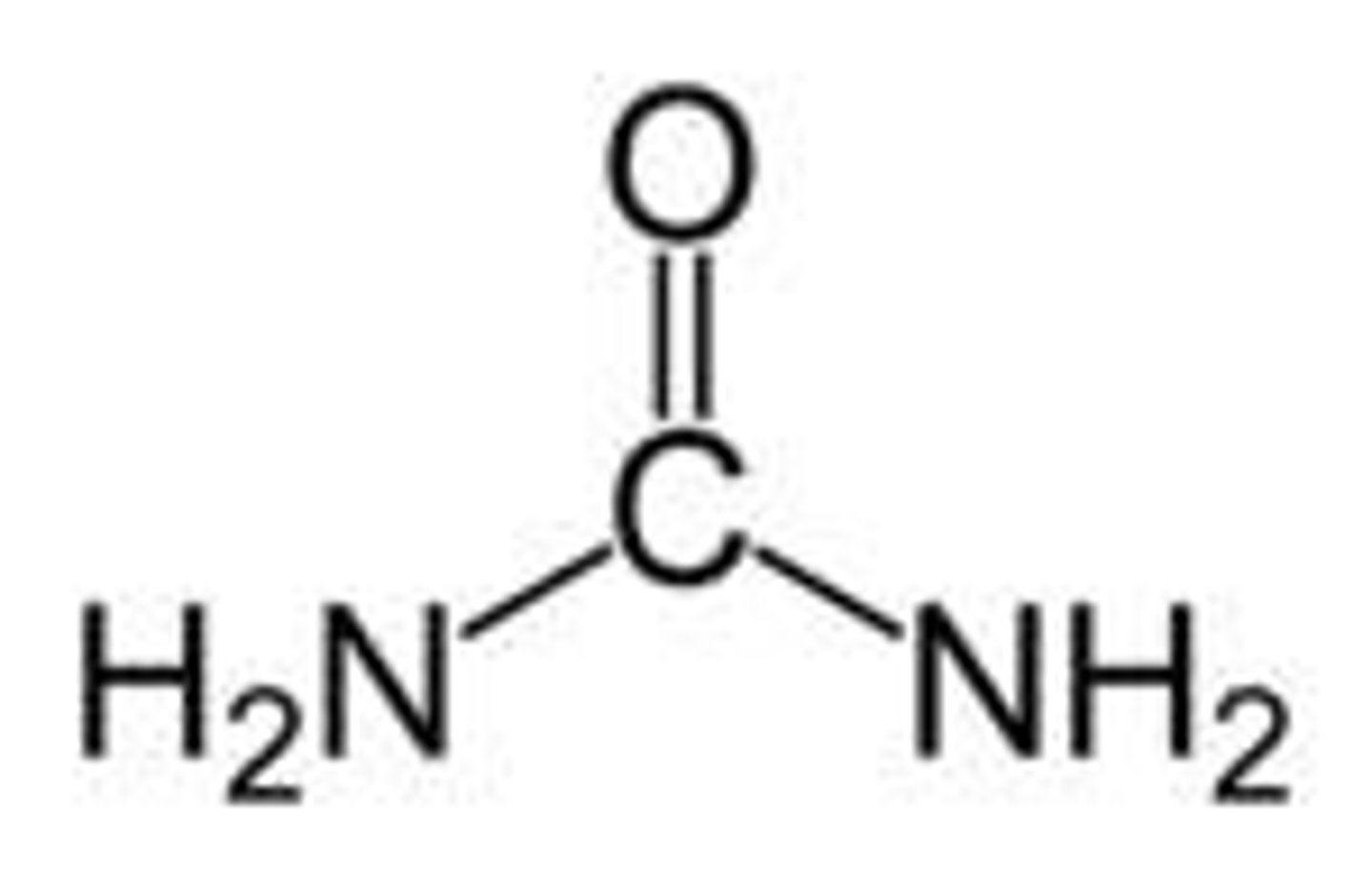
urea
azide
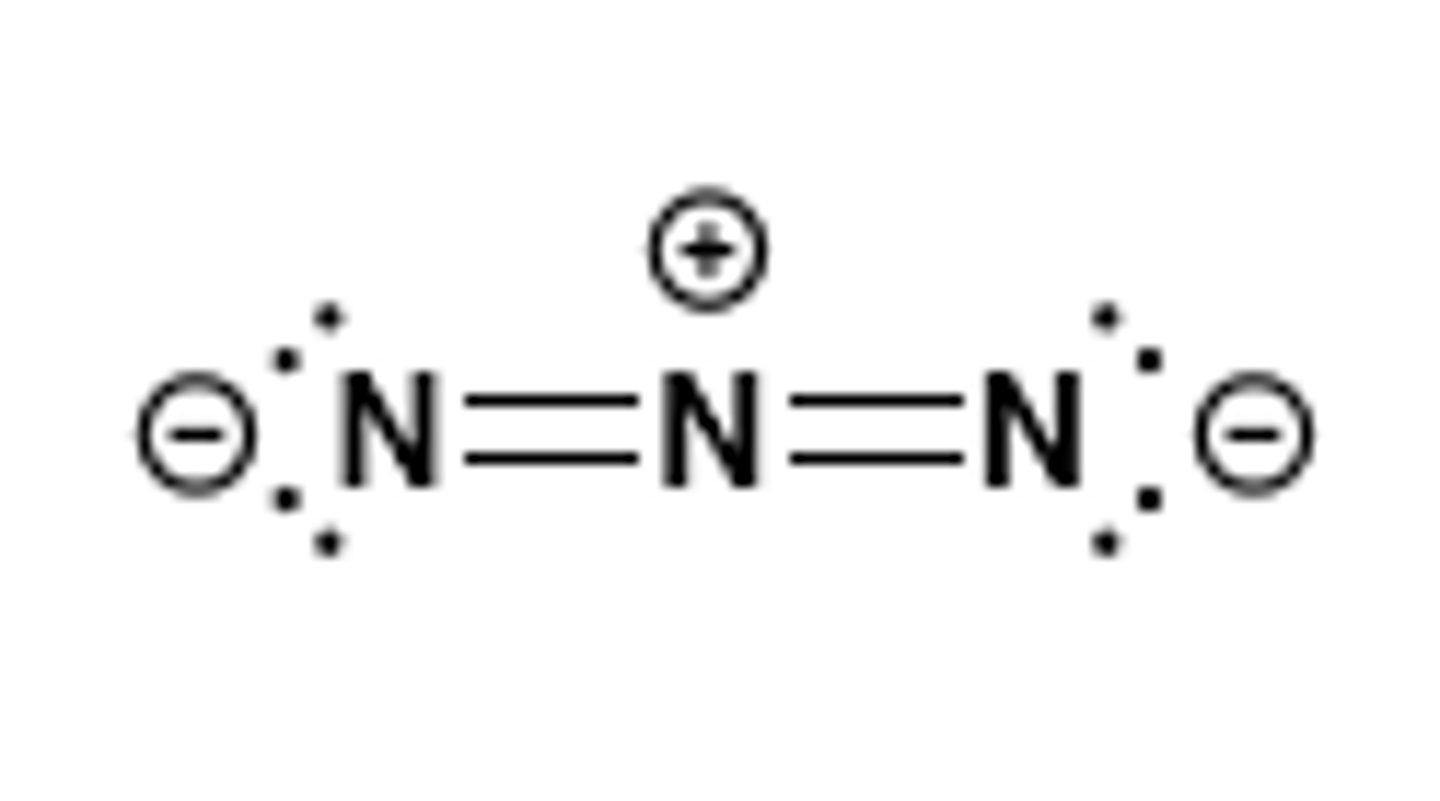
Which of the following elements is MOST likely to be a strong nucleophile?
A. Hydroxide ion
B. Water
C. Ethanol
D. Tert-butanol
A.