(unfinished) 2.2.1 Electron structure
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is a shell?
Shells are essentially energy levels, the energy increases as the shell number does
How can the maximum number of electrons in a shell be calculated?
2n² where n is the principal quantum number (shell number)
Define orbital
A region around the nucleus that can hold up to 2 electrons with opposite spins
What is the shape of the s-orbital and how many are found in each shell?
spherical, 1 in each shell from n=1
What is the shape of the p-orbital and how many are found in each shell?
dumbbell, 3 in each shell from n=2
What is the shape of the d-orbital and how many are found in each shell?
various, 5 in each shell from n=3
define sub-shell
orbitals of the same type within a shell
in which order do the subshells fill?
generally, starting from the lowest shell, orbitals fill the s, p, then d orbitals.
an exception is the 3p/4s subshell in which the 4s is filled first. this is as 3d is at a higher energy level due to the overlap of the shells as a result of the shells getting closer together as you go further from the nucleus
in which order do the orbitals fill?
orbitals of the same energy level are occupied singly before pairing
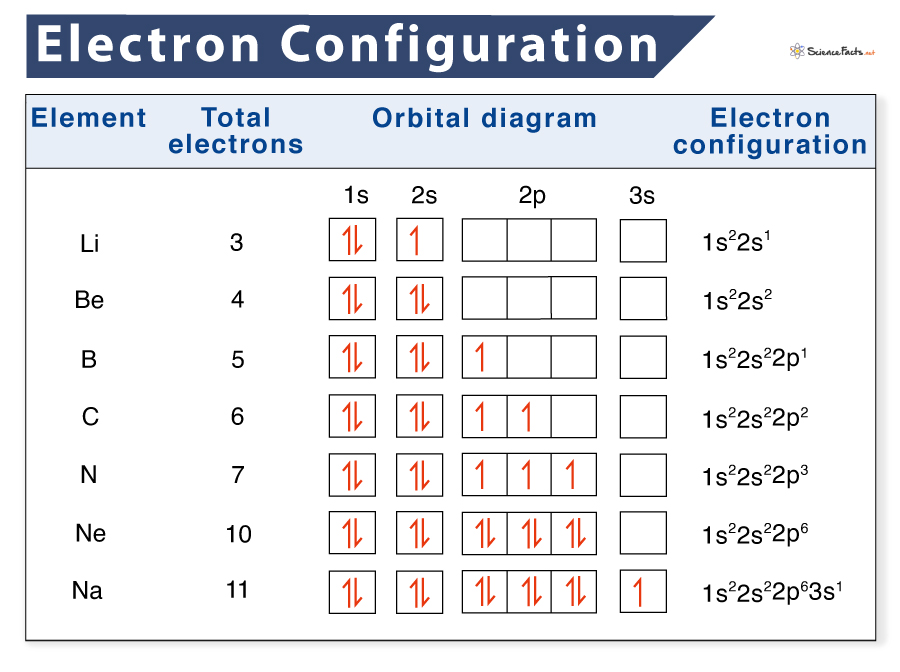
how do we use subshell notation