L1. Industrial use of m/o, streptokinase and insulin production
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
What are the main pharmaceuticals produced by m/o?
Dextrans
Nutraceuticals
Iron-chelating compounds
Streptokinase
Human proteins: insulin
What are fine chemicals produced by m/o?
Enzymes / Proteins
Biocatalysis of amino acids, vitamins
What are dextrans, what bacteria produces them and what are they used for?
Pharmaceuticals with Glucose polymers (15 kDa-20 MDa), medium has a high carbohydrate content, with its weight being strain-dependent
Produced by LAB - lactic acid bacteria e.g. Leucosnostoc spp. and are used as plasma substitutes to maintain & restore blood volume as well as application to burns/ulcers to absorb fluid.
How can the weight of dextrans be adjusted?
By acid hydrolysis or adding short templates to the fermentation medium that influence polymerization.
What are examples of vitamins produced by m/o?
Riboflavin: Yeast
Propionibacteria: Vitamin B12, used in the treatment of pernicious anaemia
What are examples of amino acids produced by m/o?
Corynebacteria: Glutamate, lysine
form the ingredients in infusions solutions
What are examples of organic acids produced by m/o?
Aspergillus niger: Citric acid, used in food production as well as acting as an anticoagulant
What m/o produces Fe chelating agents?
Siderophores produced by m/o & fungi which are compounds that bind to Fe, aiding in its solubility & Fe acquisition
Streptomyces pilosus produces Desferrioxamine, used to treat Fe poisoning / overload diseases like haemochromatosis - has a carbonate region
Paracoccus denitrificans produces Parabactin an anticancer
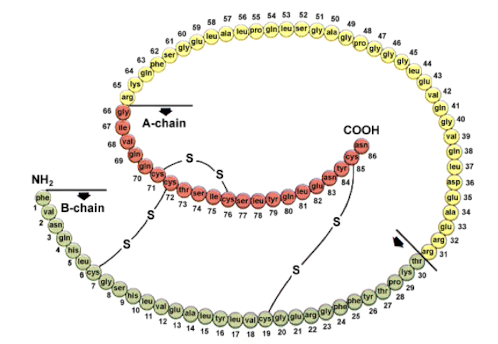
What is streptokinase?
useful blood clot dissolving protein ‘enzyme’ produced by haemolytic streptococci, that activates plasminogen a.k.a. zymogen (inactive) → plasmin (active, a protease dissolves clots), used to treat MIs and thrombosis
How does plasmin reduce the ½ life of streptokinase?
It is an active protease that dissolves blood clots, where its presence leads to the degradation of streptokinase, thereby reducing its effective duration in the bloodstream.
How can the ½ life of streptokinase be improved?
By glycosylation (addition of sugar residues) to the streptokinase protein, which can enhance its stability and prolong its presence in the bloodstream.
This glycosylated version can be expressed in a Pichia pastoris expression vector
What streptococcal species is recombinant streptokinase produced in?
Streptococcus equisimilis H46A
Recombinant streptokinase
What is the natural reason for streptococci to produce streptokinase?
To facilitate tissue invasion and spread during infections by breaking down blood clots, overcoming host defences and allowing rapid spread
Diabetes review + injectable insulin as a treatment method
Diabetes mellitus (type 1) is an autoimmune disease
Destruction of insulin-producing cells in pancreas leading to uncontrolled blood sugar levels
Renal failure, blindness, nerve damage
What are some chemical features of insulin?
Peptide hormone composed of two polypeptide chains (A and B) linked by disulfide bonds.
It regulates glucose levels in the blood and is characterised by its hydrophilic nature, which allows it to be soluble in blood.
Proinsulin needs to be proteolytically cleaved to form active insulin before it can function effectively.
What are some issues regarding isolating natural insulin in the past?
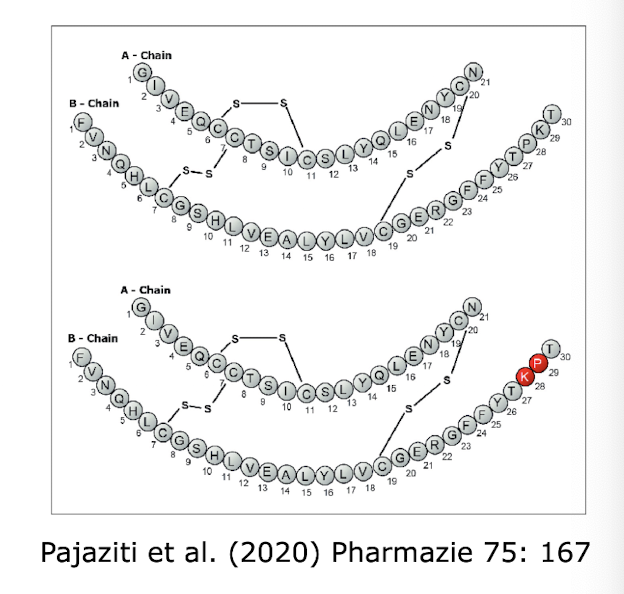
Low yield when isolated from pig pancreas in order to extract porcine insulin - which has only one AA difference (B30 Thr → Ala) from human insulin
What can be done instead to circumvent issues when extracting porcine / chemically synthesising human insulin?
Performing semi-synthesis → done via Trypsin, a reversible protease, we want to cleave the Ala from the SC → DAI (d-Ala insulin)
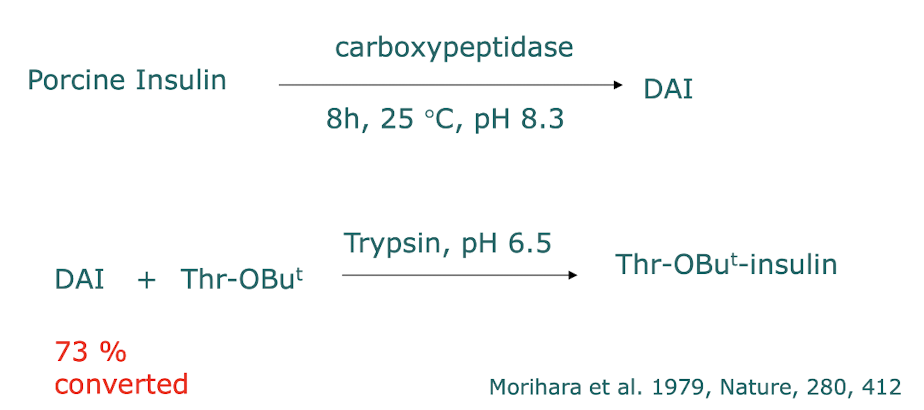
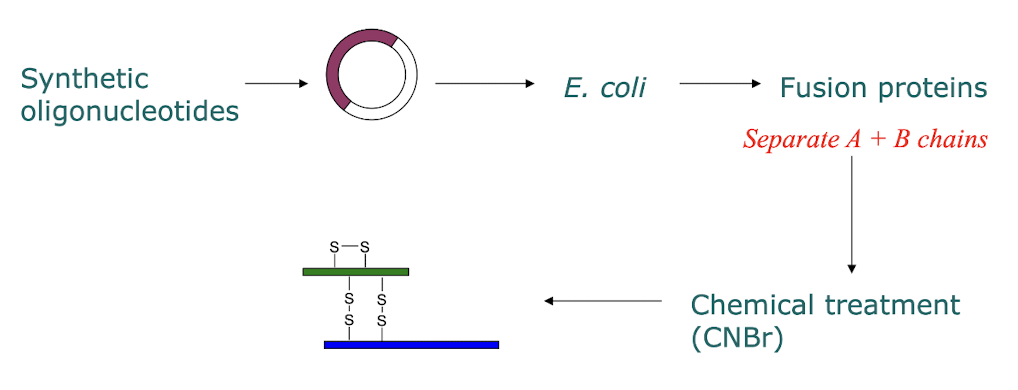
How is recombinant insulin used?
recombinant insulin using synthetic oligonucleotides, created by viewing the AA structure of the insulin, ligated the oligonucleotides to an extrachromosomal plasmid, i.e. E. coli, replicating independently, allowing for massive production of insulin
→ fusion proteins a protein of interest fused to another protein
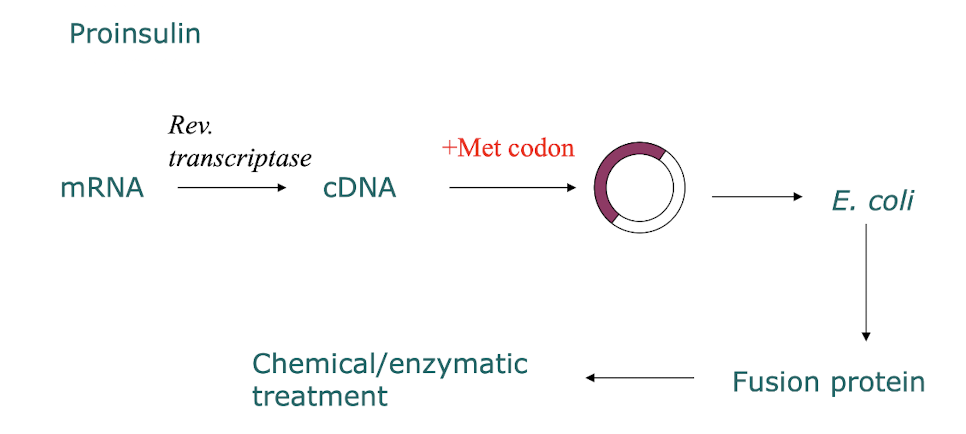
How else can recombinant insulin be made?
By altering the human mRNA encoding proinsulin (contains introns and exons NOT in prokaryotes) and using RTase to make cDNA which is then incorporated into a bacterial plasmid
What is Lispro (Humalog)?
Is a rapid-acting insulin analog created by swapping the positions AA residues in the β chain i.e. proline and lysine in the insulin molecule, allowing for quicker absorption and onset of action and preventing insulin aggregation into inactive hexamers