Human Physiology: An Integrated Approach, 8/e, Chapter 2
1/111
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
112 Terms
Stanly Miller
(1953) did a simple experiment to prove Aleksander Oparin and John Haldane (100 yrs ago) correct about how life might have arisen on earth (atmosphere - hydrogen, water, ammonia, and methane); he found amino acids had formed in the flask creating molecules associated with living creatures from nonliving inorganic precursors
human body mass makes up what three elements
oxygen (O), carbon (C), and hydrogen (H)
organic molecules
molecules that contain carbon and hydrogen (Ch 2, 16); a molecule is formed when two or more atoms are chemically linked
biomolecule
organic molecules associated with living organisms (Ch 2) Four major groups (macro or large molecules): carbohydrates, lipids, proteins, and nucleotides; Most contain C, H, & O; Basic functions is energy and building block
molecules that the body uses for energy and cellular components:
carbohydrates, lipids, and proteins
components of Genetic material:
DNA and RNA
ATP (adenosine triphosphate)
carries energy in a form the cell can use
cyclic AMP (adenosine monophosphate; cAMP)
regulate metabolism
lipids
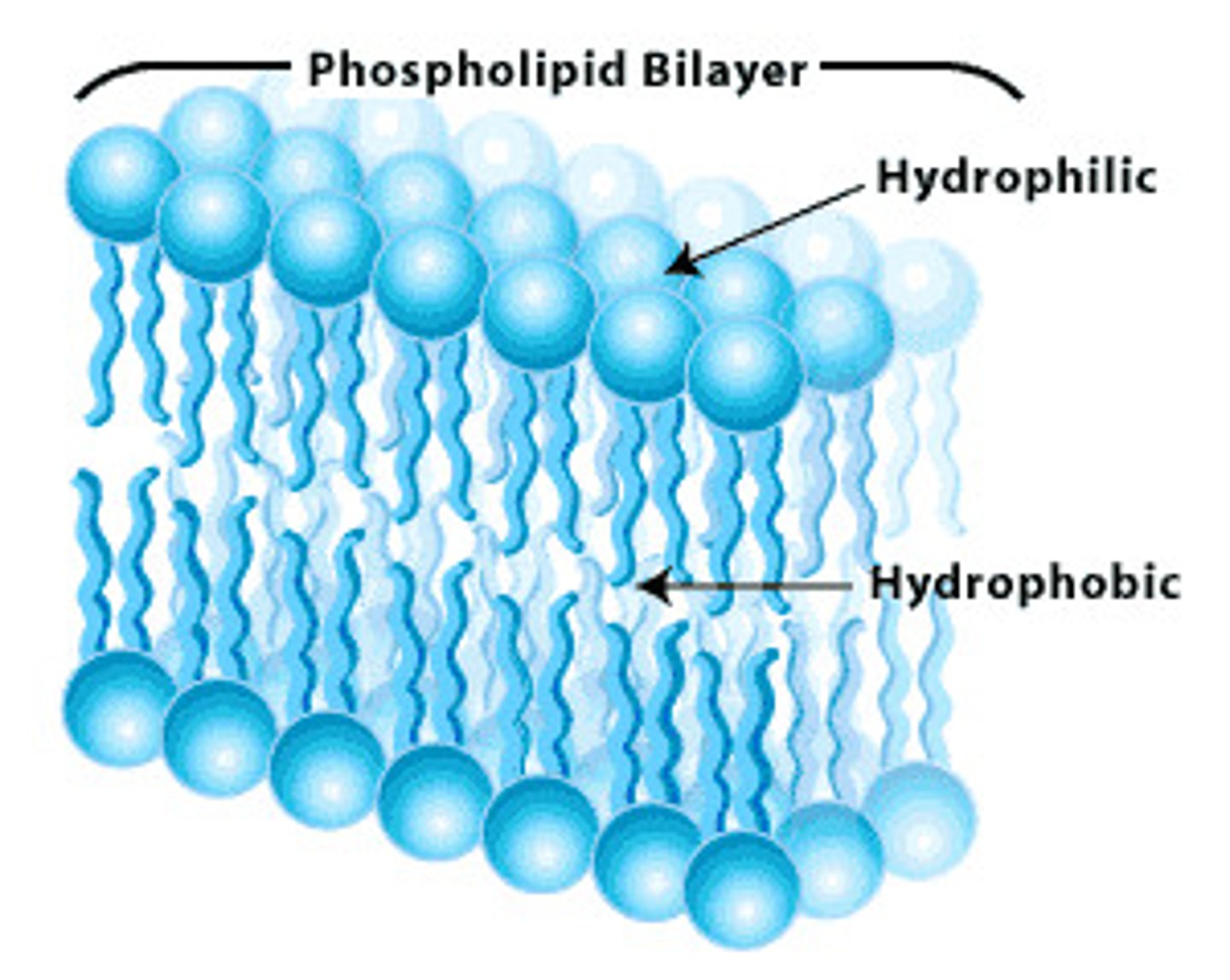
mostly carbon and hydrogen; the backbone of glycerol and 1-3 fatty acids; dietary fats: Triglycerides- saturated vs. unsaturated (unsaturated is the presence of double bonds between adjacent carbon atoms in a fatty acid); Cholesterol (important in cell membrane structure), steroids: sex hormone estrogen & testosterone that also have cholesterol base; Phospholipids: cell membrane component (two fatty acids bonded to glycerol and phosphate): have a polar and non-polar end
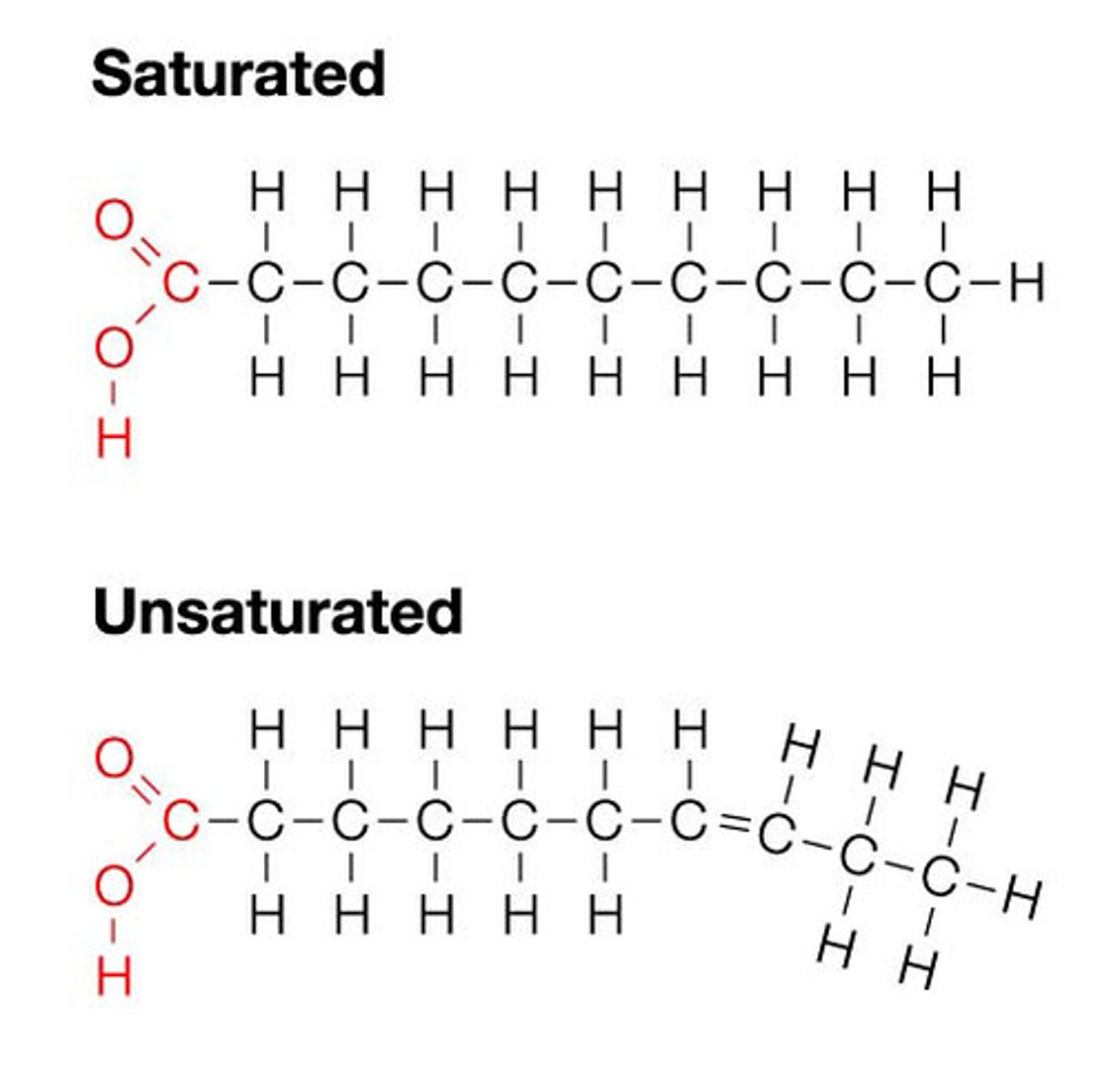
proteins and nucleotides contain
nitrogen in addition to carbon, hydrogen, and oxygen
carbohydrates
primarily carbon, hydrogen (water) and oxygen, in ratio CH*2O - for a carbon there are two hydrogens and one oxygen; includes sugars and starches; most abundant biomolecule, important energy source in human diet (glucose is the most important metabolic fuel in our body); can be divided into three categories monosaccharides (simple sugar - glucose), disaccharides (two simple sugar -sucrose, lactose, & maltose) , and complex glucose polymers called polysaccharides (many saccharides - starch, glycogen, & cellulose)
proteins
macromolecules made of two amino acids (building block molecules) that contain sulfur; they are polymers, meaning having a diverse range of functions out of all the macromolecules; largest and most complex organic molecules, have different structures: Primary -simplest, chain of amino acids (usually called a polypeptide if less than 100 amino acids in their chain but more then call it Protein), Secondary - hydrogen bonds between parts of chain turn it into helix or sheets, Tertiary - polypeptide folded into complex 3D shape either globular (wound around, rounded shape, things can bind to) or fibrous (more rope like), Quaternary - multiple subunits combined into one large 3D structure (example hemoglobin that is a huge and complex shape)
conjugated protein
molecules of protein combined with either lipid or carbohydrate (also know as biomolecule)
lipoprotein
protein combined with a lipid; found in cell membranes and in the blood; carriers for less soluble molecules such as cholesterol
nucleotides
is the fourth group that consist of DNA, RNA, ATP, and cyclic AMP; it the only macromolecule that you don't consume, it doesn't brake down and used for energy, building blocks for nucleic acids (DNA and RNA; hereditary information then formed into protein); Carry genetic information, aid in protein synthesis; each contains a sugar (D), phosphate and one of five bases: adenine, cytosine, guanine, thymine, and uracil
glycosylated molecules
molecules to which a carbohydrate has been attached
glycoprotein
molecule that is a combination of carbohydrate and protein
glycolipid
molecule that is a combination of carbohydrate and lipid
polymers
large molecules made up of repeating units
functional group
groups of atoms that tend to move from molecule to molecule as a single unit
amino group
chemical formula is NH2
carboxyl group
chemical formula is COOH
electrons biological roles
1. negatively charged, covalent bonds 2. Ions 3. High-energy electrons 4. Free radicals
electrons
small, negatively charged subatomic particles that orbit around the nucleus of each atom; have important biological roles: formation of bonds, creation of ions, high-energy electrons, and free radicals
protons
positively charged, determine element's atomic number (never changes)
neutrons
neutral, no charge (can vary); Isotope-same element, differing number of neutrons
atomic mass
protons plus neutrons
molecule
two or more atoms sharing electrons = covalent bonds
covalent bond
bonds created by two atoms that share one or more pairs of electrons; strong bonds require energy to make or break; single, double, and triple bonds; polar and nonpolar molecules
double bond
bonds formed when two atoms share two pairs of electrons
polar molecule
molecules that develop regions of partial positive and negative charge when one or more atoms in the molecule have a strong attraction for electrons; Example is water (Covalent bonds between different atoms sometimes share electrons unequally. The stronger atom has slightly negative charge, the weaker atom has slightly positive charge)
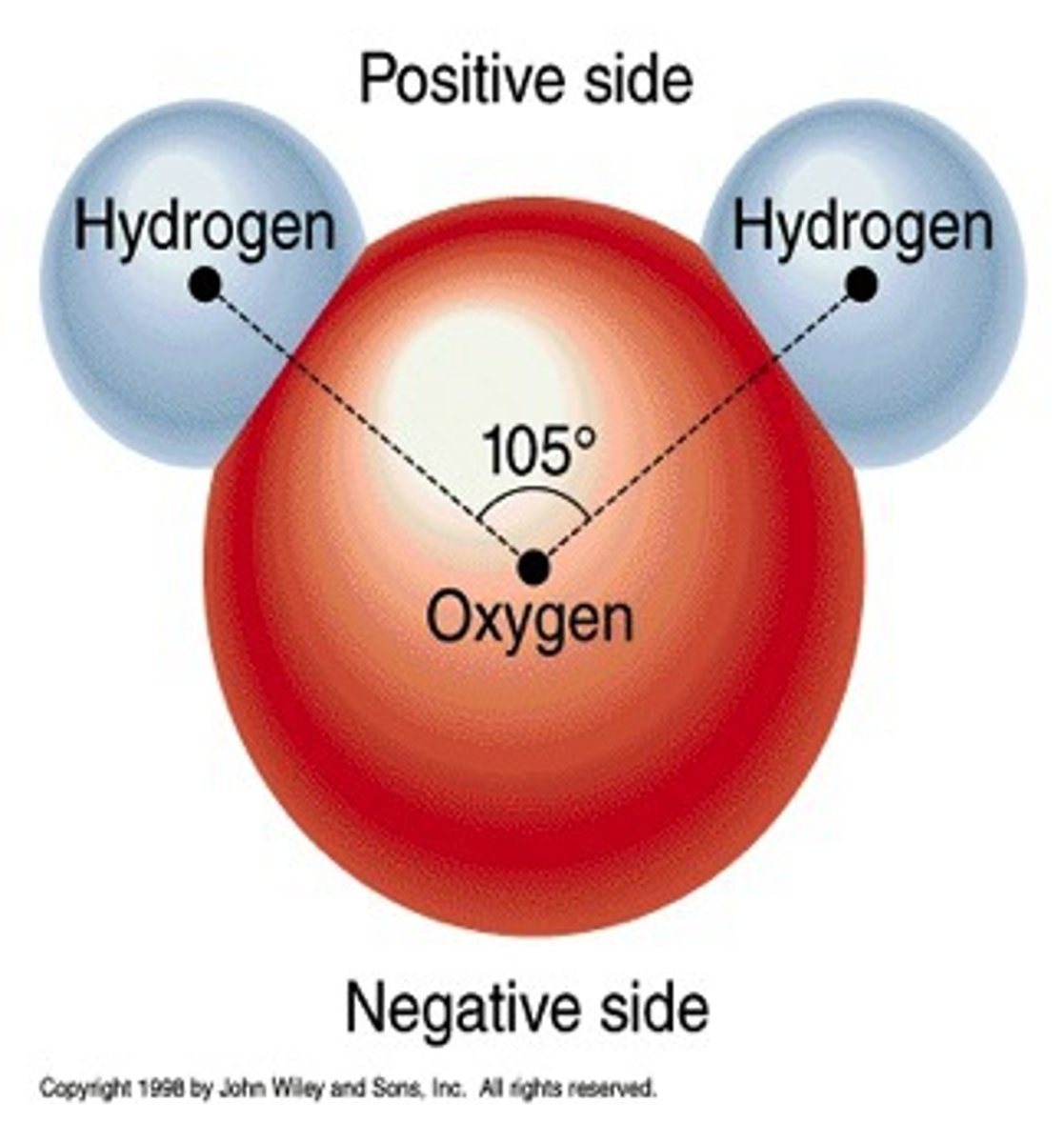
nonpolar molecule
a molecule whose electrons are distributed so evenly that there are no regions of partial positive or negative charge; Example is Oxygen (A covalent bond that shares electrons "equally")
anions
negatively charged ions; Chloride (C), Bicarbonate (HCO3-), Phosphate (HPO4^2-), Sulfate (SO*4^2-)
cation
positively charged ions; Sodium (Na+), Potassium (K+), Calcium (Ca^2+), Hydrogen (H), and Magnesium (Mg^2+)
ions
charged by atoms; Cations - lost electrons and positively charged and Anions - gained electrons and negatively charged
ionic bonds
(also known as electrostatic attractions) a bond between ions attracted to each other by opposite charge (Ch 2); atoms gain or lose electrons; opposite charges attract
hydrogen bond
Weak attractive forces between the slightly positive hydrogens (H) and the slightly negative oxygen (O) or nitrogen (N); formed between adjacent water molecules (because of their polarity); important for protein and nucleic acid structure; basis of surface tension
surface tension (of water)
the intermolecular hydrogen bonds between molecules of water at the surface; causes water to form spherical droplets when falling or to bean up when spilled onto a nonabsorbent surface.
Van der Waals forces
weak attractive force that occurs between two polar molecules or a polar molecule and an ion
solubility
the ease with which a molecule or gas (solute) dissolves in a solvent: the more easily a substance dissolves, the higher its solubility; Hydrophilic -soluble in water, polar molecules, & ionic molecules and Hydrophobic - not soluble in water, nonpolar molecules (e. g. fats)
hydrophilic
molecules that dissolve readily in water; Example, if salt (NaC1) crystals are placed in water, polar regions of the water molecules disrupt the ionic bonds between sodium and chloride, which causes the crystals to dissolve
hydrophobic
molecules that do not dissolve readily in water, non-polar; Example: Cholesterol will not dissolve unless it binds to special water-soluble carrier molecules. High-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol, the "good" and "bad" forms of cholesterol associated with heart disease.
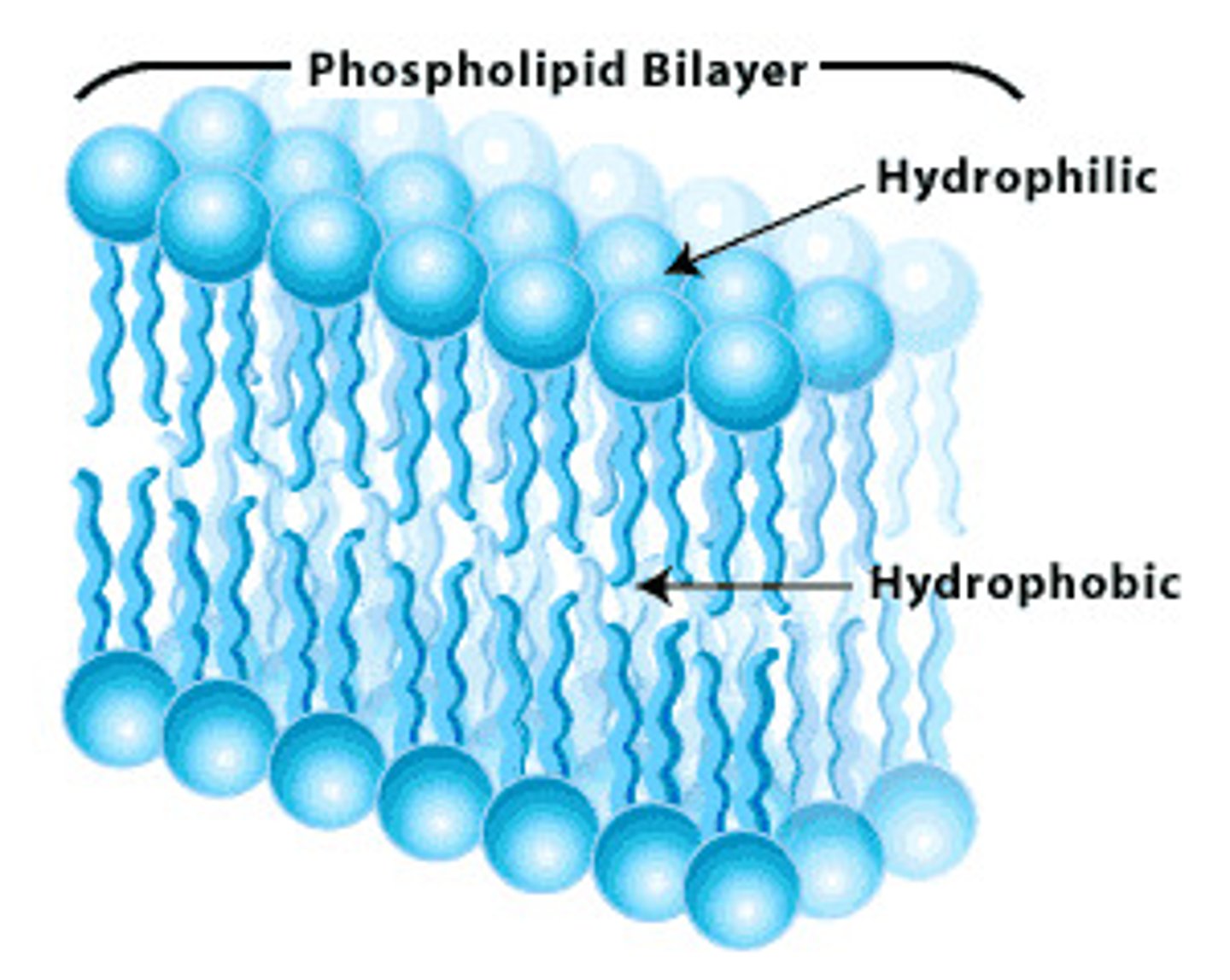
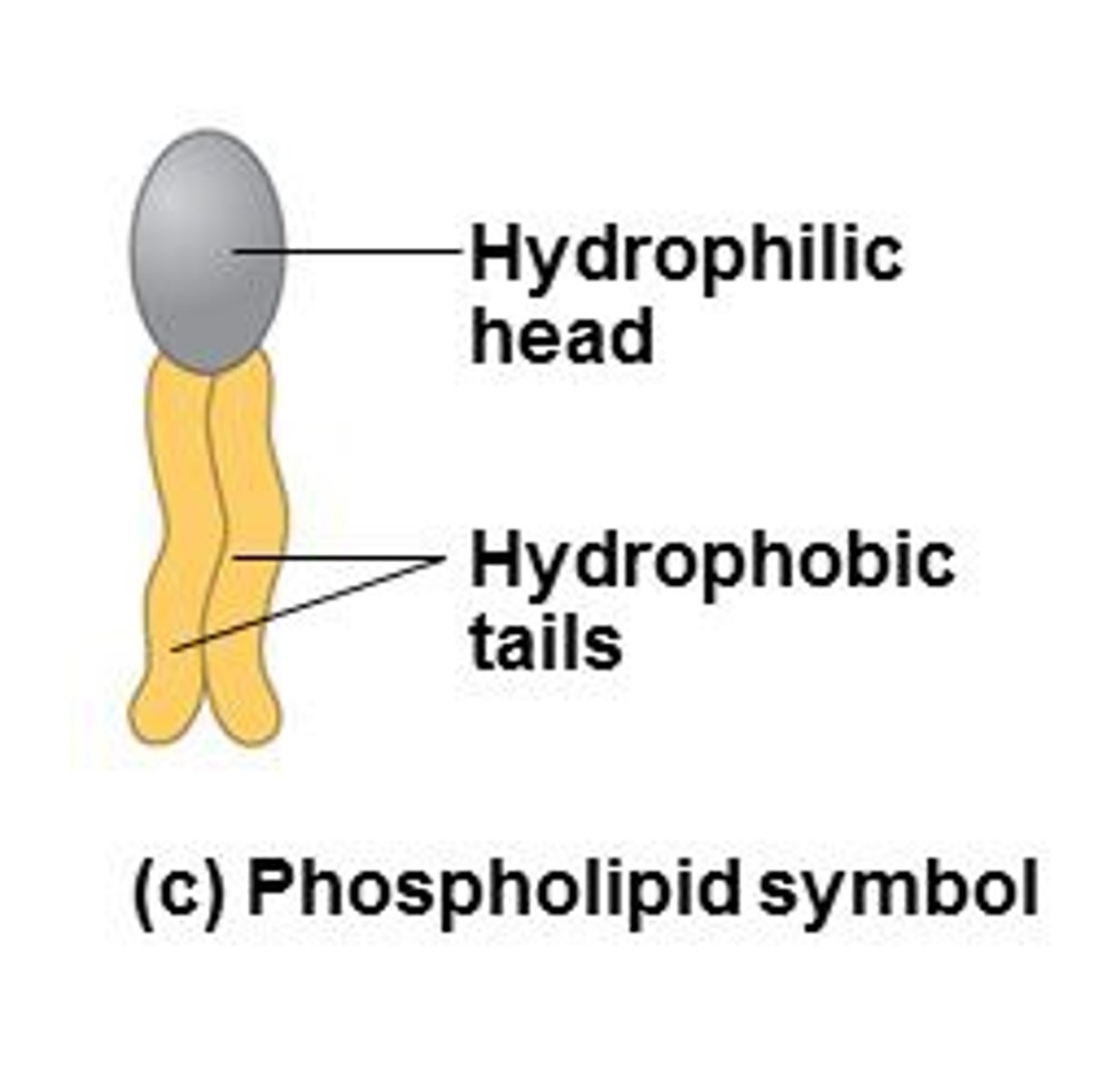
phospholipids
a molecule that is a constituent of the inner bilayer of biological membranes, having glycerol and fatty acids as key components that have a polar, hydrophilic head and a nonpolar, hydrophobic tail.
primary structure
the sequence of amino acids in the peptide chain
secondary structure
spatial arrangement of amino acids in the chain
A-helix
(alpha-helix) spiral
B-sheets
Zigzag shape
tertiary structure
the third level of protein structure; the overall, three-dimensional shape of a polypeptide due to interactions of the R groups of the amino acids making up the chain.
globular proteins
spherical, water-soluble proteins.
fibrous proteins
long, insoluble, structural proteins.
acid
molecule that ionizes and contributes an Hydrogen Ion (H+) to a solution
buffer
a molecule that moderates changes in pH
cell aggregation and adhesion
glycosylated proteins and glycosylated lipids in cell membranes create a "sugar coat" on cell surfaces
soluble proteins (fall into seven broad categories)
1. Enzymes 2. Membrane transporters 3. Signal molecules 4. Receptors 5. Binding proteins (like oxygen-transporting protein hemoglobin and the cholesterol binding protein such as LDL) 6. Immunoglobulins (like antibodies) 7. Regulatory proteins (like transcription factors bind to DNA and alter gene expression and protein synthesis)
enzymes
protein catalysts that speed up reactions by lowering their activation energy
receptors
proteins that bind signal molecules and initiate cellular responses
antibodies
help protect the body from foreign invaders and substances
binding site
region of an enzyme or transport protein to which the substrate binds
ligand
a molecule that binds specifically to another protein molecule complementarity, usually a larger one.
substrate
The ligand that binds to an enzyme or a membrane transporter
specificity
the ability of an enzyme or receptor to bind to a particular molecule or a group of closely related molecules; Example, enzymes known as peptidases bind polypeptide ligands and break apart peptide bonds, no matter which two amino acids are joined by those bonds. Aminopeptidases also break peptide bonds but are more specific.
protein binding
when the ligand and protein come close to each other, noncovalent interactions between the ligand and the protein's binding site allow molecules to bind
induced-fit model of protein-ligand activity
the active site changes shape to fit either substrate or product molecules
affinity
the degree to which a protein is attracted to its ligand
reversible binding
indicated by the symbol of the double arrow. P is Protein and L is Ligand. When they are put together or bound it is PL or Protein-ligand complex. The bounding is exactly equal to the rate of unbinding or dissociation: P+L=>PL and P+L<=PL
The ratio is called equilibrium consitant K*eq
equilibrium constant K*eq
the ratio of product concentrations to reactant concentrations at equilibrium, with each concentration raised to a power equal to the number of moles of that substance in the balanced chemical equation
Example: K*eq = [PL]/[P][L]
Protein-ligand complex is in the state of equilibrium with Protein plus the Ligand (square brackets [ ] around the letters indicate concentrations of molecule)
law of mass action
for a reaction at equilibrium, the ratio of substrates to products is always the same; also known as the Le Chatelier's Principle (Ch 2, 18, 20)
dissociation constant (Kd)
the reciprocal of the equilibrium constant; Example: large Kd indicates low binding affinity of the protein for the ligand with more P and L remaining in the unbound state and small Kd means higher value for [PL] relative to [P] and [L], small Kd indicates higher affinity of the protein for the ligand
competitors between ligands
the ligand molecule compete for the binding sites of the proteins
agonist (contestant)
molecules that combine with a receptor mimic a response (Ch 2, 6, 7, 8, 11); Example: competing ligands that mimic each other's actions; the ability of agonist molecules to mimic the activity of naturally occurring ligands has led to the development of many drugs
isoform
related forms of a molecule
cofactor
an ion or inorganic or nonprotein organic molecule required for activation of protein before binding site will become active and bind to ligand; many enzymes will not function without their cofactors; Ca^2+, Mg^2+, Fe^2+
modulator
a factor that influences either protein binding or protein activity
antagonist
(also called inhibitors) one substance opposes the action of another
competitive inhibitor
molecules that bind to the active site of the enzyme, preventing substrate binding (Ch 2, 5, 7)
allosteric modulator
binds to an enzyme away from the binding site and change the shape of the active site
covalent modulator
atoms or functional groups bind to proteins and affect their activity
penicillium mold (antibiotic penicillin)
discovered by Alexander Fleming (1928); it inhibited bacterial growth in a petri dish. In 1965 researchers figured out how the antibiotic works. Penicillin is an antagonist that binds to a key bacterial protein by mimicking the normal ligand. Because penicillin forms unbreakable bonds with the protein, the protein is irreversibly inhibited. Without the protein, the bacterium is unable to make a rigid cell wall. Without a rigid cell wall, the bacterium swells, ruptures, and dies.
allosteric modulators
bind reversibly to a protein at a regulatory site away from the binding site, and by doing so change the shape of the binding site
competitive inhibitor
a substance that reduces the activity of an enzyme by binding to the enzyme's active site in place of the substrate. A competitive inhibitor's structure mimics that of the enzyme's substrate.
covalent modulators
atoms or functional groups that bind covalently to proteins and alter the protein's properties
up-regulation
increase in protein number or binding affinity that increases the response of the target cell (Ch 2, 6)
down-regulation
decrease in protein number or binding affinity that lessens response (Ch 2, 6, 7)
saturation
all active sites on a given amount of protein are filled with substrate and reaction rate is maximal; enzymes, membrane transporters, receptors, binding proteins, and immunoglobulins
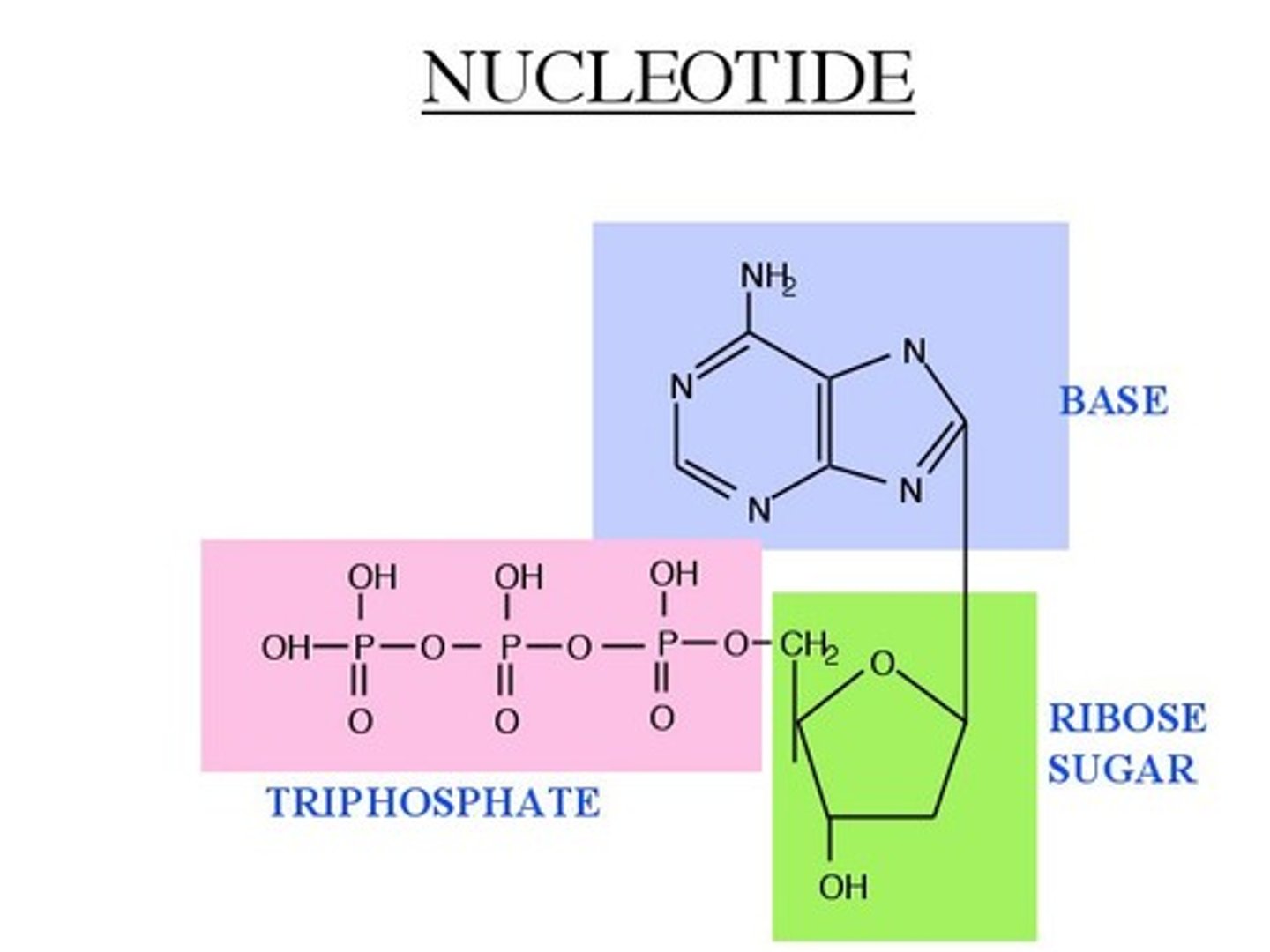
nucleotides & nucleic Acids
a nucleotide is made up of five-carbon sugar, a nitrogenous base, and a phosphate group, they transfer energy and are part of genetic material;
nucleic acids are polymers of units called nucleotides;
because purines are larger than pyrimidines, space limitations always pair a purine with a pyrimidine;
guanine (G) forms three hydrogen bonds with cytosine (C);
adenine (A) forms two hydrogen bonds with thymine (T) or uracil (U);
a nucleotide containing the base cytosine (C) would base pairs with a nucleotide containing the base guanine (G);
a nucleotide containing the base thymine (T) would base pair with the nucleotide containing the base adenine (A).
solutions
dissolved in solvent, biological solutions are water-based = aqueous; Example: Ions (solute) dissolved in water (solvent)
expressions of Volume
as liters (L) or milliliters (mL);
deci (d) 1x10^-1, milli (m) 1x10^-3, micro (μ) 1x10^-6, nano (n) 1x10^-9, and pico (p) 1x10^-12
Acids, Base/Alkaline, and pH
Free H can participate in hydrogen boding and Van der Walls forces; can change a molecule's shape or conformation; pH measurements of the concentration of free H concetration: pH<7 is acidic and pH>7 is basic/alkaline; Acids are molecules that release H when dissolved in water; Buffer moderates changes in pH - bicarbonate anion, HCO*3, is an important buffer in the human body
acids
any molecule that contributes free H+ to a solution
bases
any molecule that decreases H+ concentration by binding with free hydrogen ions
pH
power of hydrogen; the measure in grams (g) of Hydrogen Ion Concentration (H+) in body fluids is measure in terms of pH
protein Interaction
function as: Enzymes, Transport proteins, Signal molecules (e.g. hormones), Receptors, Binding proteins, Immunoglobulins (a.k.a antibodies), and regulatory proteins
denatured protein
a protein whose structure has been changed by physical or chemical agents; lost its unique 3-D shape; temperature and pH values outside of the protein's normal range can cause loss of shape, which results in loss of function
bonding behavior
determined by the number and arrangement of electrons
isotope
Atoms of the same element that have different numbers of neutrons
HCI (hydrochloric acid) in the stomach
acid breaks down food in your stomach, it is good to have enough acid to break down your food easily
- acid also kills any bad organisms that entered your digestive system via your food; in acidic solution it donates its H+ and shows the pH lower then 7
covalent double bond
share two pairs of electrons (a total of four)
polar covalent bond
a chemical bond that has unequally shared electron pairs
Atomic number
an element equals the number of protons in an atom of that element (or in the nucleus)
No shared pairs of electrons in these bond
ionic