IMED1003 - The Urea Cycle (L20)
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Summary so Far
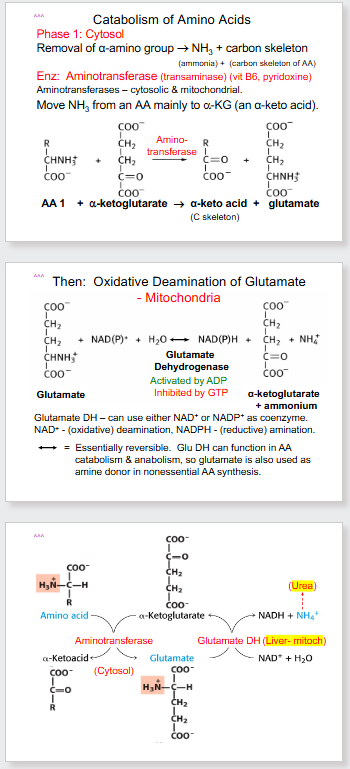
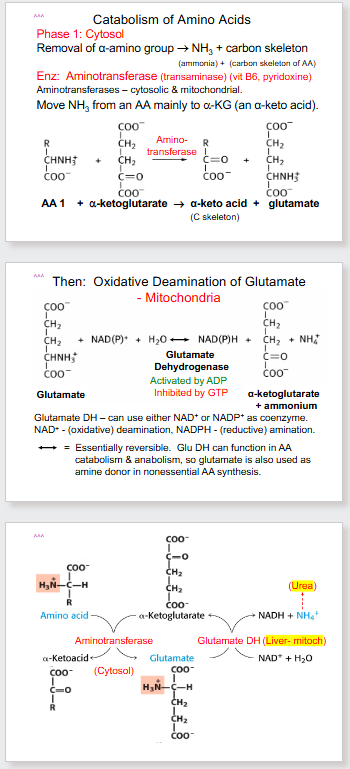
- the name aminotransferase is exactly how it sounds. It is the transfer of amino group from one substance to another.
- another name for glutamate is glutamic acid (which is why you dont want it in the blood, can cause acidosis)
Why Ammonia is toxic
- tolerated conc of ammonia in blood is around 50 micromoles per litre
- neurlogical issues ensue above this
- these include ataxia, and if ammonia isn't cleared from blood you can go into a coma
What u need to know about Urea Cycle
- dont need to do all ractions, but you need to know the ATP requirements becuase this is a vital pathway we undertake
- also need to know the overall reaction
- some of the key enzymes
- where the enzymes are active
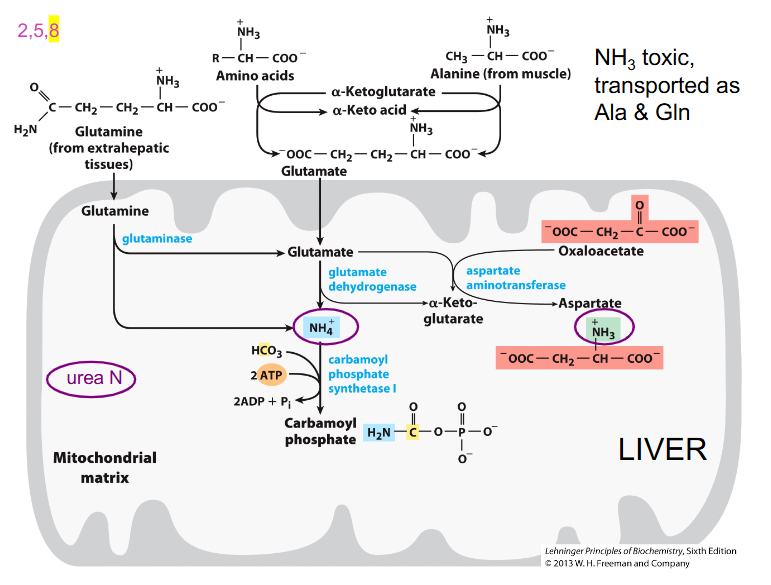
NH3 made in tissues is toxic
- must maintain low blood [NH3]
- two "ways" to move N safely in blood: Gln or Ala
.
- the reason we use these ones is because they are uncharged and will not affect tonicity of sol
- muscles make alanine, most other tissues make glutamine
- these amino acids can then travel in the blood and go to the liver safely
- glutamate differs from glutamine because glutamine has an extra amino group
![<p>- must maintain low blood [NH3]</p><p>- two "ways" to move N safely in blood: Gln or Ala</p><p>.</p><p>- the reason we use these ones is because they are uncharged and will not affect tonicity of sol</p><p>- muscles make alanine, most other tissues make glutamine</p><p>- these amino acids can then travel in the blood and go to the liver safely</p><p>- glutamate differs from glutamine because glutamine has an extra amino group</p>](https://knowt-user-attachments.s3.amazonaws.com/86a4c7d9-c041-4274-962f-b0ca6461b624.png)
How the muscles make use of alanine
- the muscles make alanine and shunt it through blood to the liver
- in the liver alanine will have amino group removed so it becomes pyruvate
- pyruvate is glucogenic and hence the liver can make glucose from it
- the muscles then absorb that glucose thats produced
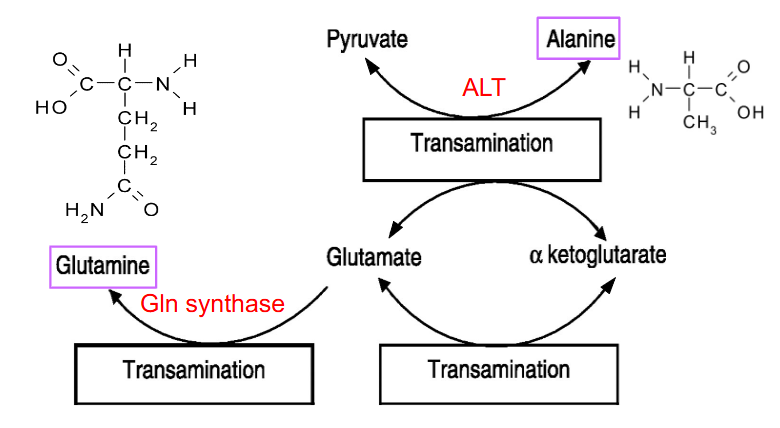
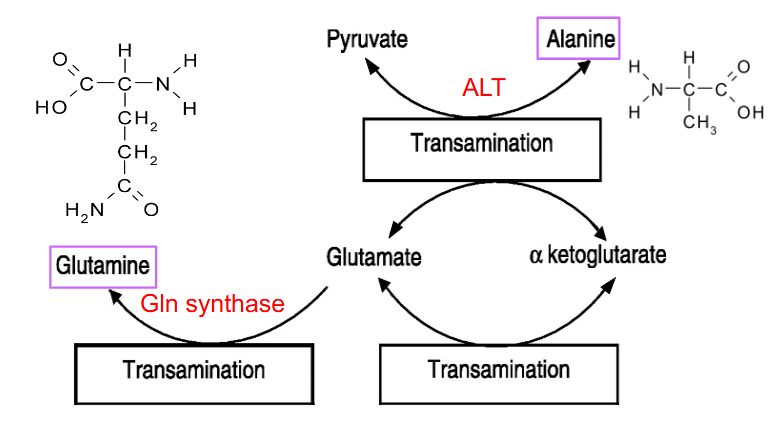
Enzymes for amine movement
- Alanine to Pyruvate: alanine amino transferase (ALT) (also reversible)
- Glutamate to glutamine: glutamine synthase (Gln synthase)
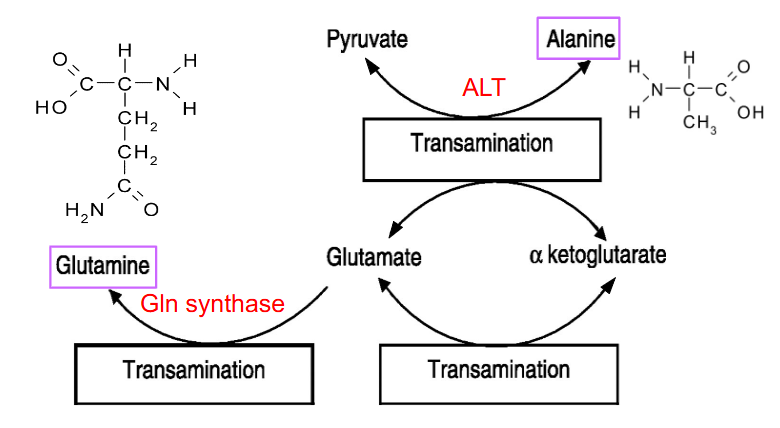
Glutamine
- made in many tissues, NH3 transferred to Glu by Gln synthase -> Gln --> blood
- in liver, glutaminase removes NH3 to reform Glu - can be processed to alpha-ketoglutarate
- Then ALT Ala -> Pyruvate, used in gluconeogenesis
.
- glutamine synthase is expressed in basically all tissue. the conversion to glutamine requires ATP, but now glutamine can be safely transported to liver
- glutaminase reverses the above reaction, stripping off the amino group and forming glutamate
- basically we have safely moved an amino group from the body cells to the liver, where now the NH3 can form urea
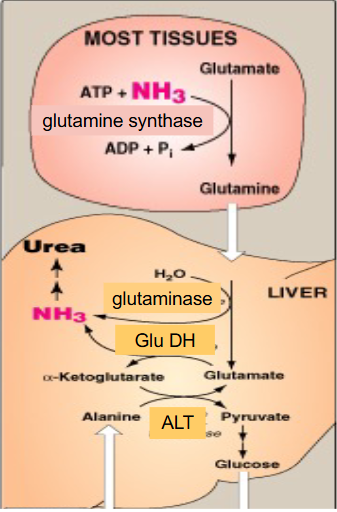
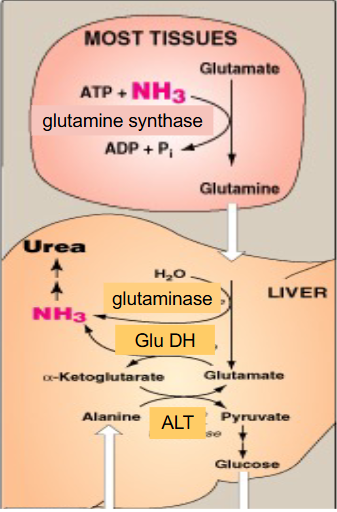
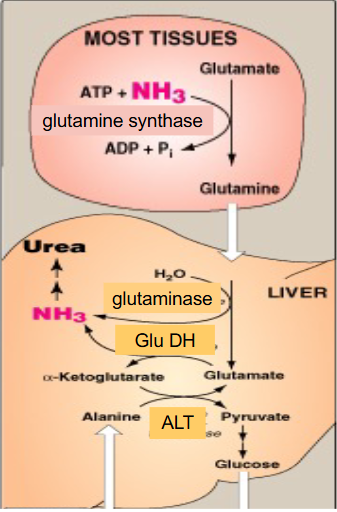
What the liver can do with leftover glutamate
- glutamate is now present after the NH3 was removed from glutamine by glutaminase
- liver has high levels of Glutamate Dehydrogenase which means we can strip off the second amino group from glutamate
- the second amino group could have come from when another amino acid added amino group to alpha-ketoglutarate to form glutamate
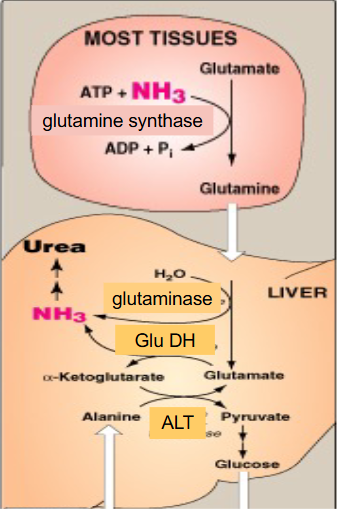
Alanine Delivery
- the alpha-ketoglutarate and glutamate pairing, combined with alanine in the presence of ALT can form glucose
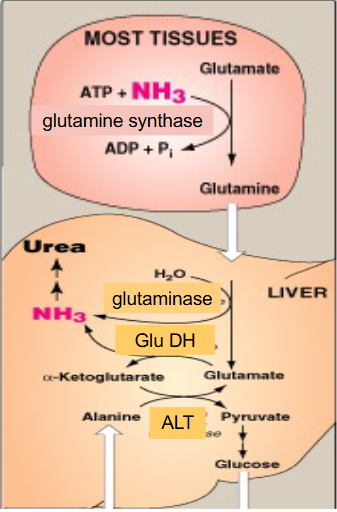
Transport of NH3 to liver EQUATIONS
DIAGRAM ON SLIDE 8
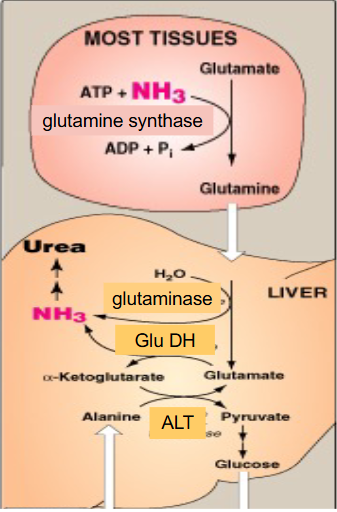
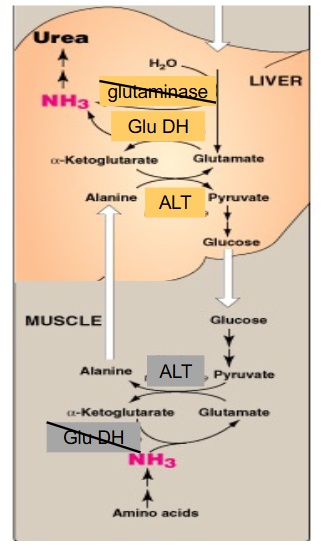
Alanine Amino Transfer to Liver
- in muscle, AA transamination to Glu. ALT produces Ala from pyruvate and Glu
- Ala --> blood
- in liver, ALT reverses this to give Glu and pyruvate - use in gluconeogenesis and return glucose to muscle = glucose-alanine cycle (NOT THE CORI CYCLE)
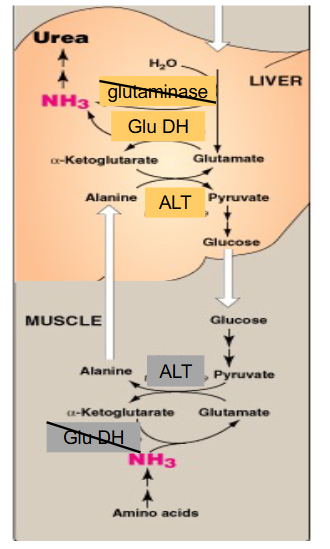
Nitrogen Excretion
- urea cycle - liver and kidney
- Cylical, so reutilise intermediates
- Major route for removal of ammonia - AA catabolism
- NH3 toxic - need low blood [NH3], transported from tissues to liver as Gln and Ala
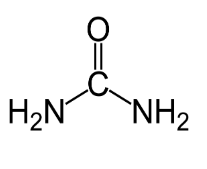
- Urea - soluble, excreted in urine.
- Not all urea excreted, lots processed in gut by bacteria
- Urea N - from NH3 and Asp
![<p>- urea cycle - liver and kidney</p><p>- Cylical, so reutilise intermediates</p><p>- Major route for removal of ammonia - AA catabolism</p><p>- NH3 toxic - need low blood [NH3], transported from tissues to liver as Gln and Ala</p><p>- Urea - soluble, excreted in urine.</p><p>- Not all urea excreted, lots processed in gut by bacteria</p><p>- Urea N - from NH3 and Asp</p>](https://knowt-user-attachments.s3.amazonaws.com/8e632331-6974-4236-998d-9a3f04a7dd92.png)
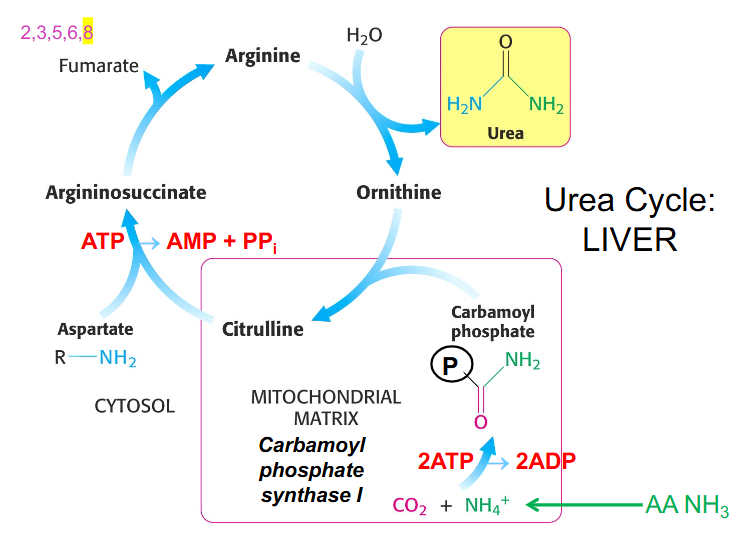
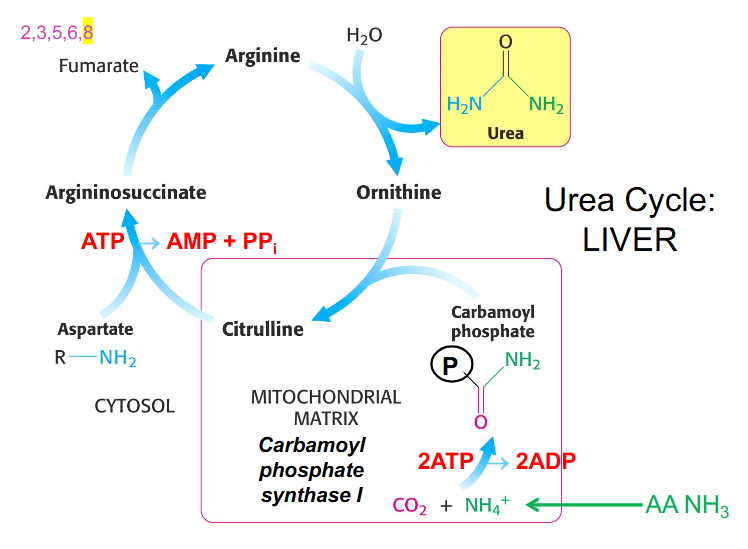
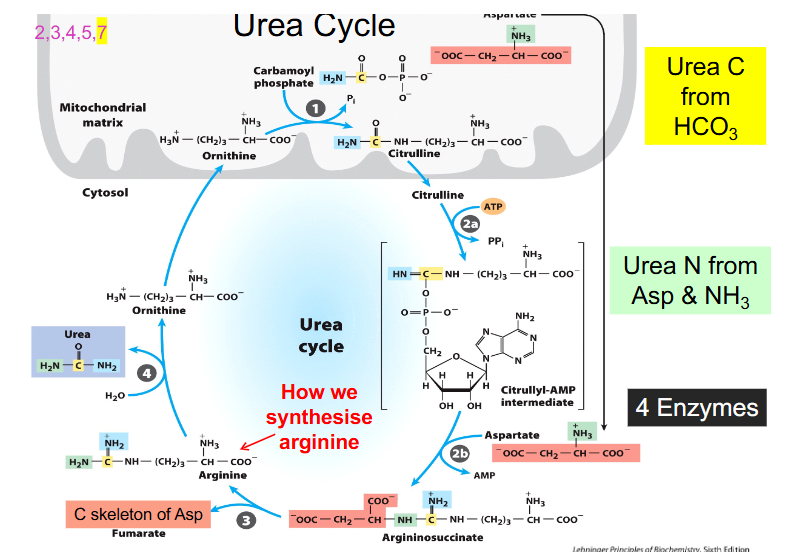
Urea Cycle Components
- ornithine is the starting point
- ornithine is derived from arginine and functions in urea cycle as a carrier of nitrogen
- citrulline is another different type of amino acid that is a nitrogen carrier
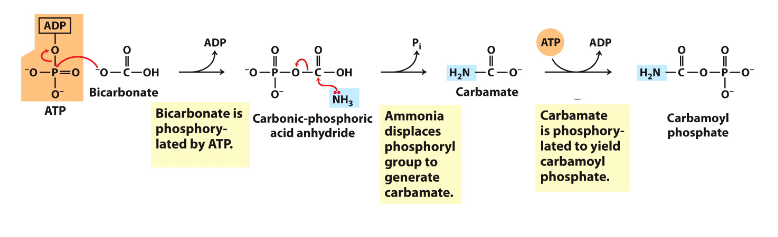
- Carbamoyl phosphate synthase 1 makes carbamoyl phosphate by combining CO2 and NH4+ or bicarbonate and ammonia and uses 2 ATP to synthesise the activated carrier of amino group
- ornithine then binds to carbamoyl phosphate in the mitochondria and forms citrulline
- citrulline moves back out into cytosol and combines with aspartate and our second amino group of urea using 2 ATP equivalents to form argininosuccinate
- from argininosuccinate we produce arginine and we release 4 carbon fumarate
- from fumarate we can get OAA (TCA Cycle)
- from arginine we can reform ornithine
ALL PAIRS U NEED TO KNOW SO FAR
- Alaine and Pyruvate
- Aspartate and OAA
- Glutamate and Alpha-Ketoglutarate
- IN THE UREA CYCLE: Aspartate and Fumarate
UREA CYCLE + TRANSPORT DIAGRAM
- glutamate cant travel in the blood becuase its acidic which is why it travels as glutamine
- glutamate dehydrogenase releases amino group
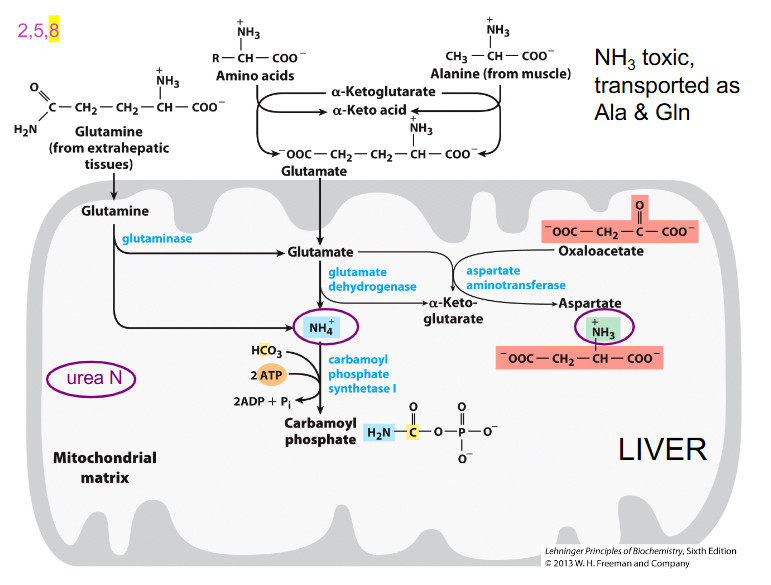
Making Urea - 1N from NH3
- Glutamate is a principal amine donor to other AA
- Free ammonia released from glutamate is converted to urea for excretion
- Carbamoyl phosphate synthase 1 captures free ammonia - mitochondrial matrix
- First step (nitrogen-acquisition) of urea cycle, requires 2ATP, multistep
DONT NEED TO KNOW THE DETAILS
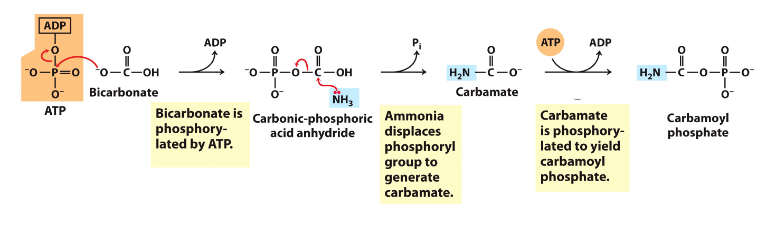
Making Urea - 1N from Asp
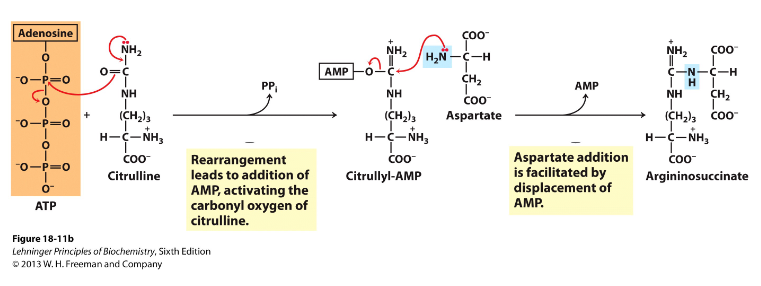
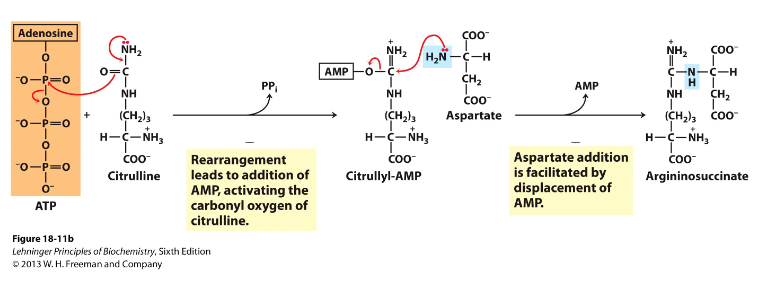
- Second nitrogen-acquiring reaction - cytosol
- Enzyme = argininosuccinate synthase, uses 2ATP equivalents, 2 step process
- Citrulline's O is activated using ATP, then aspartate added and AMP displaced, making argininosuccinate
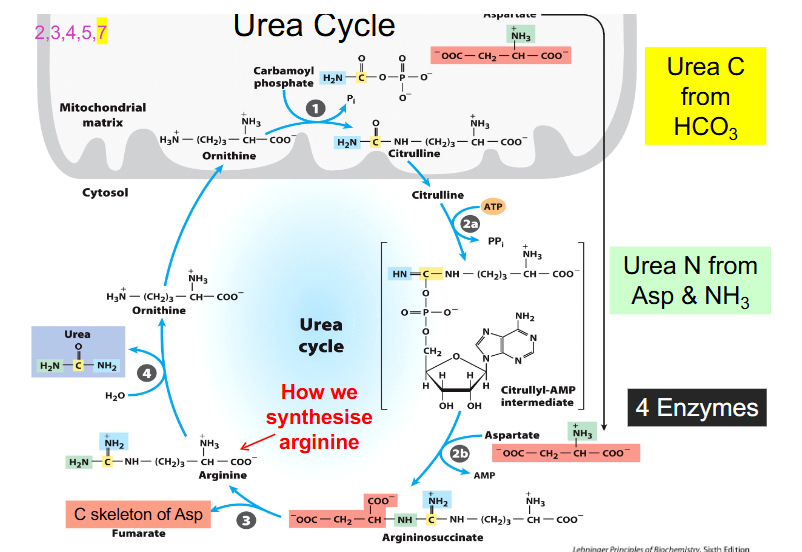
FULL UREA CYCLE DIAGRAM
- enzyme you have to remember is called ornithine transcarbamoylamylase (because it adds carbamoyl group to ornithine)
- this is the most common issue that people have with their urea cycle is that they either dont make enough or its not functioning well enough
- the enzyme that cleaves argininosuccinate to arginine is called argininosuccinase
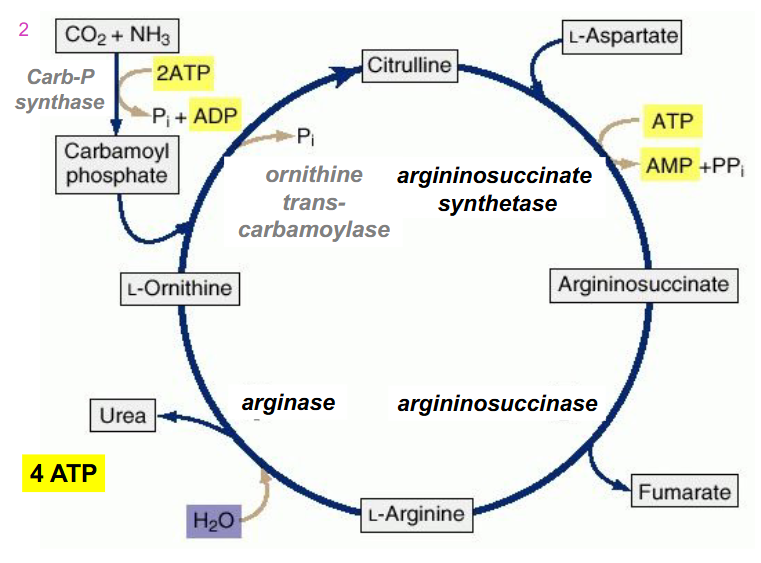
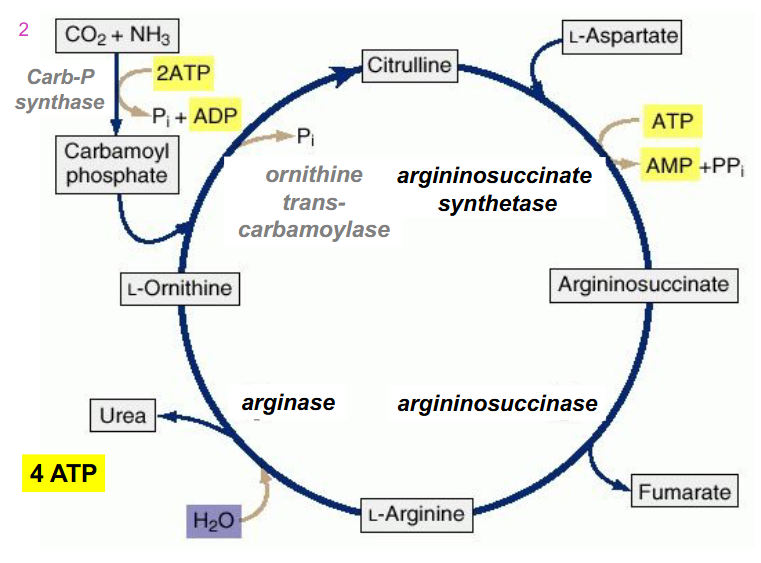
SIMPLIFIED REACTIONS OF UREA CYCLE
DIAGRAM ON SLIDE 17
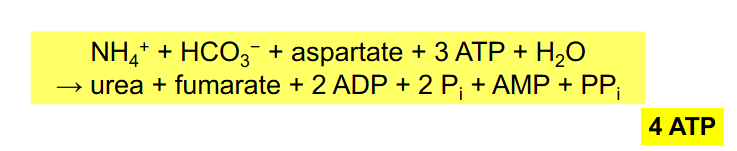
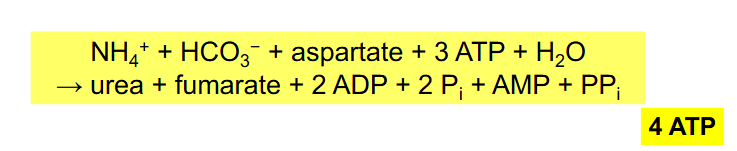
Overall Reactions of Urea Cycle
NH4+ + HCO3- + aspartate + 3ATP + H2O --> urea + fumarate + 2ADP + 2Pi + AMP + PPi
- equivalent of 4 ATP used
- NEED TO RMBR OVERALL RXN
Regulation of Urea Cycle
- expression of urea cycle enzymes increases when needed, usually enough enzymes to cope with a reasonable amount of AA catabolism, but need more if:
- High protein diet or starvation - protein being broken down for energy
- get upregulation of enzyme gene transcription and translation
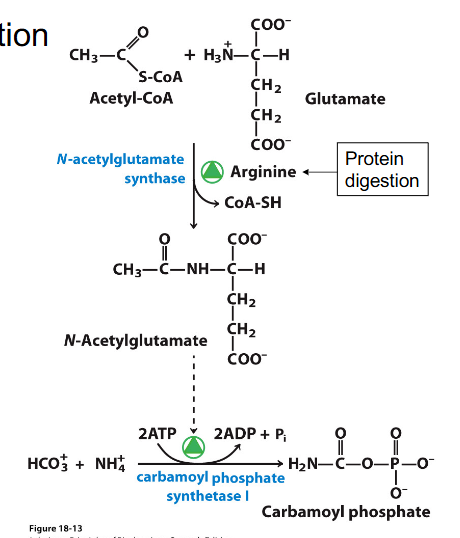
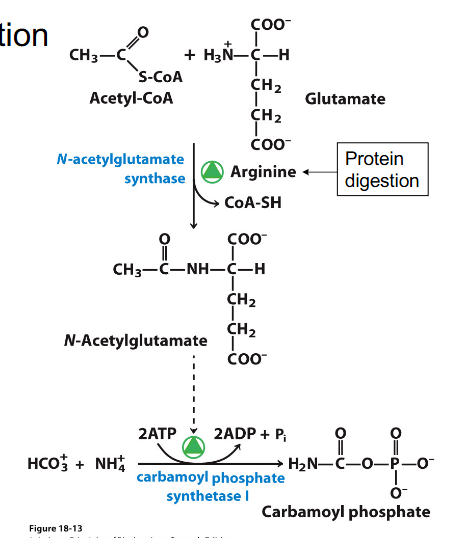
Regulation of Urea Cycle through chemicals
- Allosteric regulation of carbamyol phosphate synthase 1 (CPS1)
- activated by N-acetylglutamate (precursor of ornithine)
- so, after a high protein meal, get activation of enzymes for urea production and supply of glutamate to make the activator or CPS1
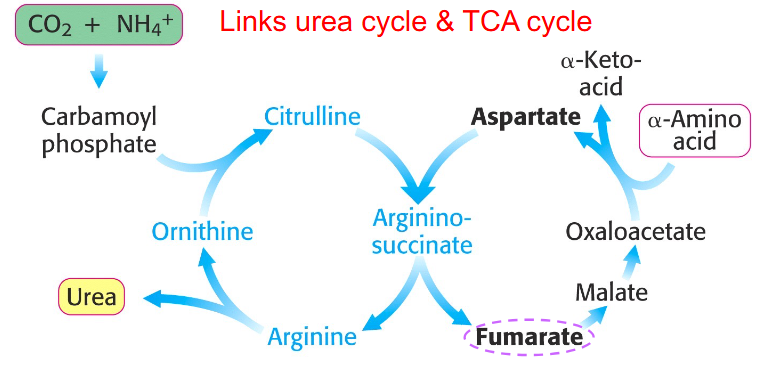
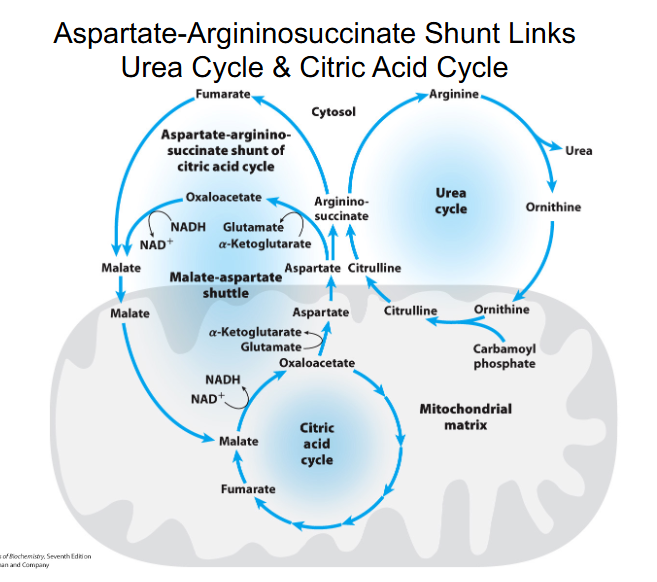
aspartate-argininosuccinate shunt
NH4+ + HCO3- + aspartate + 3ATP + H2O --> urea + fumarate + 2ADP + 2Pi + AMP + PPi
- there is communication between the amino and carbon skeleton part
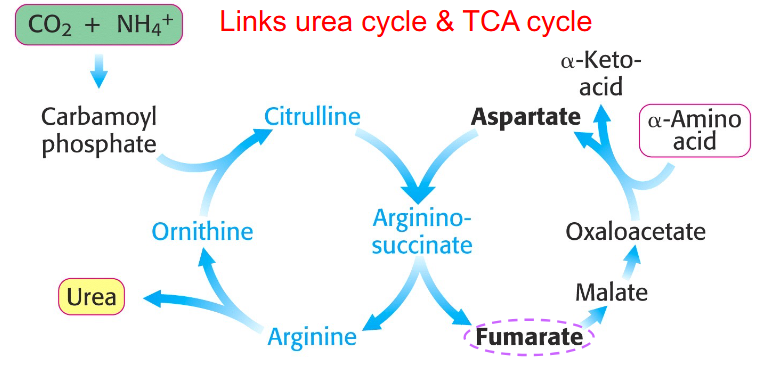
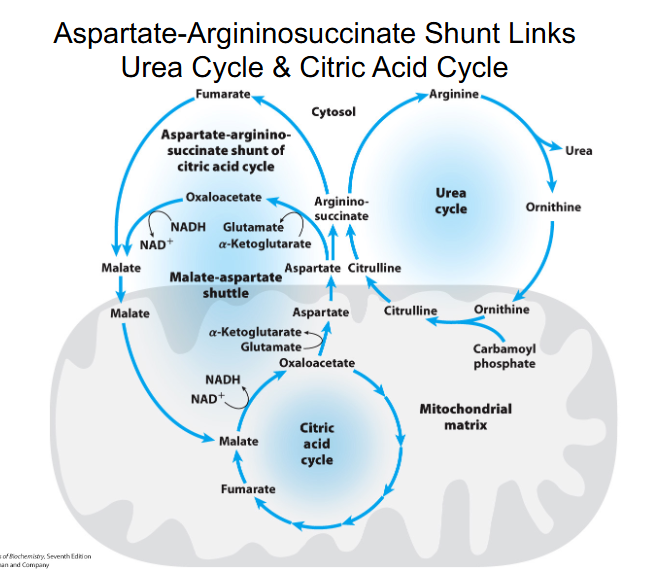
Aspartate-Argininosuccinate Shunt Links Urea Cycle and Citric Acid Cycle
DIAGRAM ON SLIDE 22
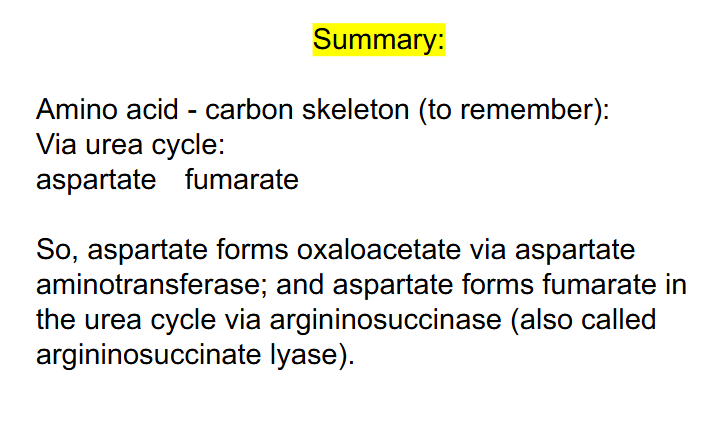
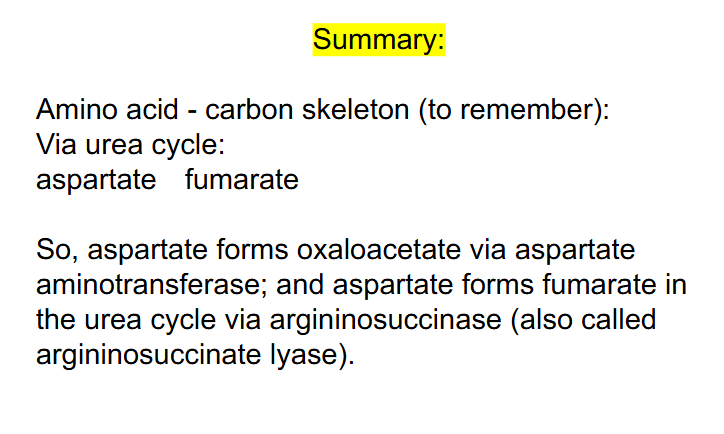
SUMMARY
DIAGRAM ON SLIDE 23