GEN CHEM 1
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
75 Terms
THE TRIPLET REPRESENTATION
Macroscopic, microscopic, symbolic
Pure substances
an be an element or a compound
Element
the simplest type of substance with unique physical and chemical properties
consists of only one type of atom
Compound
a substance composed of atoms of two or more elements that are chemically combined
properties are different than those of its elements
Molecule
a structure that consists of two or more atoms that are chemically bound together and thus behaves as an independent unit
• Can be elements or compounds
Mixture
a group of two or more elements and/or compounds that are physically intermingled
Heterogenous Mixture
has one or more visible boundaries between the components
Homogenous Mixture
as no visible boundaries because the components are mixed as individual atoms, ions, and/or molecules
Is also called a solution
Aqueous Solution
Solutions in which water is the solvent
Chemical Property
A property that is that is associated with a change in chemical composition
Examples: flammability, pH
Chemical Change
A change of matter that results in a change in its chemical composition
Examples: rusting of metal, lighting a match, taking antacid for heartburn
Physical Property
A property that is that is not associated with a change in chemical composition
Examples: physical state/phase, density, color
Physical Change
A physical change occurs with a change in state/phase
Examples: melting ice, boiling water, tearing paper, sanding wood
Solid
has a fixed shape and volume
May be hard or soft, rigid or flexible
Liquid
as a varying shape that conforms to the shape of the container, but a fixed volume
Has an upper surface
Gas
has no fixed shape or volume and therefore does not have a surface
Metric Prefixes
Tetra 10^12. deci 10^-1
Giga 10^9 centi 10^-2
Mega 10^6 milli 10^-3
Kilo 10^3 micro 10^-6
Hecto 10^2 nano 10^-9
Deca 10^1 pico 10^-12
7 Base SI Units
Length- meter (m)
Mass- kilogram (kg)
Temperature- kelvin (K)
Time- second (s)
Electric Current- ampere (A)
Amount of Substance- mole (mol)
Luminous Intensity- candela (cd)
Derived Units
Volume- m³
Density- kg/m³, g/cm³
Gases- g/mL
Accuracy
how close a measurement is to the true value
Precision
how close repeated measurements are to each other
Significant Figures
Recorded digits (including the estimated one) of a measurement
They reflect how “certain” (precision) we are in the measurement value and help us know how “correct” (accuracy) that value is
The last digit recorded for a measurement is always estimated
The more digits a number has, the greater certainty we have in its value
Exact Number
A number with no uncertainty in its value
• 1 ft is exactly 12 in
• 1 in is exactly 2.54 cm
• 1 kg is exactly 103 g
-Contain an infinite number of significant figures
Non Exact Number
Quantities derived from measurements other than counting that ALWAYS have a certain level of uncertainty
• Due to the practical limitations of the measurement process
• The number of a measurement must be reported to indicate its uncertainty
The temperature outside (e.g., 72°F)
Your height or weight
The time it takes to get to school or work
The speed of a car
Non Zeros
These are always counted as significant in reported measurements
Zeros
These are sometimes significant and only in these cases:
• At the trailing end of the number AND a decimal is explicitly written
• That are “captive” between non-zeros
Unit Conversions
use a method called the Factor- Label Method
Utilizes ratios of equivalent units called conversion factors
Scientific Formulas
use mathematical equations to describe the relationships between properties
Dimensional Analysis
The general process of treating units as mathematical quantities in a calculation
Conversion Factor
is a ratio between equivalent measurements each expressed in different unit
Factor Label Method
is a blueprint for unit conversions that utilizes conversion factors to guide the cancellation of units
Postulates of Dalton’s Atomic Theory (1)
All matter consists of atoms; tiny indivisible particles of an element that cannot be created or destroyed
Postulates of Dalton’s Atomic Theory (2)
Atoms of one element cannot be converted into atoms of another element
Postulates of Dalton’s Atomic Theory (3)
Atoms of an element are identical in mass* and other properties but are different from the atoms of any other element
Postulates of Dalton’s Atomic Theory (4)
Compounds result from the chemical combination of a specific ratio of atoms of different elements
The Law of Conservation of Mass
Matter cannot be created or destroyed
The Law of Constant Composition
A specific compound is always composed of the same elements in the same mass percents
The Law of Multiple Proportions
If elements A and B react to form two compounds, the different masses of B that combine with a fixed mass of A can be expressed as a ratio of small whole numbers
Atomic Theory Today
The nucleus consists of protons and neutrons
• The nucleus contributes 99.97% of the atom’s mass but occupies only about 1 quadrillionth of its volume
• The nucleus diameter is about 20,000 times smaller than the diameter of the atom
Atomic Number
The number of protons in the nucleus of an atom
• Its value determines the identity of the atom
Neutral Atoms
Means the total positive (+) charge = total negative (-) charge
# of p+ = #e-
The atomic number (Z) also indicates the # of e–
The Periodic Table
Increasing atomic number (Z) order
• Groups: vertical columns that contain a “family” of elements
• Periods: horizontal rows that display a predictable repeating pattern of chemical properties
Isotopes
are atoms that have the same # of p+ but a different # of n0
• Have the same atomic number (Z) but a different mass number (A)
• Are atoms of the same element
Ion
is an atom (or molecule) that has lost or gained one or more electrons
Cation
Positively charged ion
Anion
Negatively charged ion
Atomic Mass
Is the weighted average of all the masses of isotopes present in a natural sample
Reported on the periodic table
Chemical Formula
consists of element symbols, numerical subscripts, and sometimes parenthesis
• Indicates the type and number of each atom/ion present in the smallest unit of a substance
7 Diatomic Elements
Hydrogen
Nitrogen
Oxygen
Fluorine
Chlorine
Bromine
Iodine
Polyatomic Elements
Phosphorous- 4
Sulfur- 8
Selenium- 8
Chemical Formula Examples (3 Models)
Structural Formula
Ball and Stick Model
Space filling Model
Empirical Formula
indicates the smallest whole-number ratio between the atoms (or ions)
Molecular Formula
Indicates the actual numbers of atoms (or ions) in a molecule
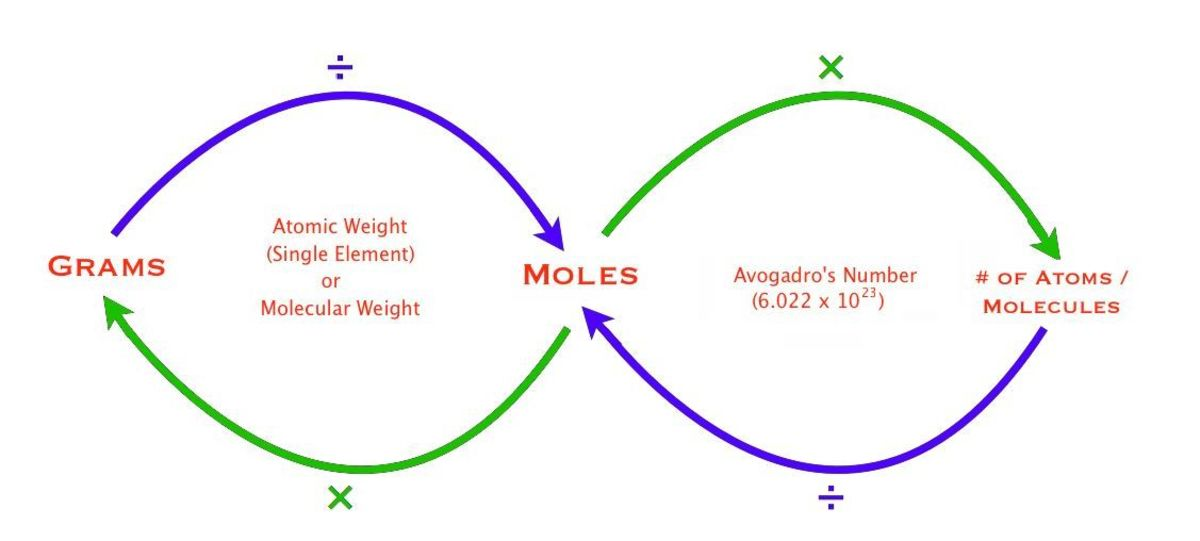
The Mole- Avogadro’s Number
the amount of a substance that contains the same number of entities as there are atoms
Formula Mass
• Sometimes called molecular mass or formula weight
• Sum of atomic masses
Molar Mass
Relates one mole of a substance to its formula mass
• Numerically equal to the formula mass
• Units are grams per mole, g/mol
Mass-Moles-Atoms
Electromagnetic Radiation Spectrum
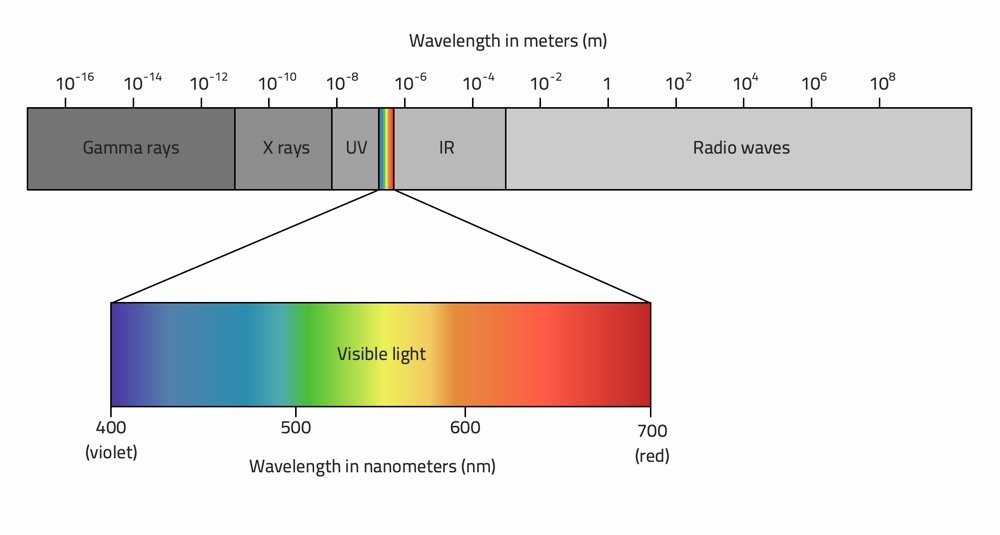
Wavelength and Frequency relationship
Inversely proportional to each other through the speed of light
Matter (particles)
Comes in chunks you can hold & weigh
• Its quantity can be changed piece by piece
• Moves in specific paths
Energy (waves)
• Is massless
• Its quantity changes on a continuous spectrum
• Light energy travels in disperse waves
The Particle Nature of Light (3 Exceptions)
Blackbody Radiation
The Photoelectric Effect
Atomic Emissions
Blackbody Radiation
when a solid object is heated to high temps, it gives off electromagnetic radiation
Example: an incandescent lightbulb
The Photoelectric Effect
when sufficient frequencies of light shines on a metal plate, a current flows
Example: solar panels
Atomic Emissions
when atoms are exited by energy they emit radiation with discrete wavelengths and not in a continuous spectra.
Example: Neon signs
Quantum Theory
the energy of a small particle is quantized – it occurs in fixed packets (photons) rather than being a continuous spectrum
Quarters, rungs on a ladder
Photon
A quantized “packet” of electromagnetic radiation
• The energy is directly proportional to its frequency through Planck’s constant.
• Has no mass and at the speed of light in a vacuum
The Particle Nature of Light
A small particle changes its energy by emitting or absorbing one or more photons of light
The energy that of the emitted or absorbed photon is equal to the difference in the energy states
Wave-Particle Duality
Light exhibits this:
It travels as an electromagnetic wave, described by wavelength, and frequency
It transfers energy in discrete packets called photons, whose energy depends on frequency
The specific behavior of light that is observed depends on how we interact with it
The Early Atomic Model
An atom consisted of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus
This description was incomplete, since an electron moving in an elliptical orbit would be accelerating and emitting radiation
The Bohr Model
Electrons occupy specific "orbits” around the nucleus, each corresponding to a fixed energy level.
Electrons can "jump" between these energy levels, but they cannot exist between them
An electron emits or absorbs a photon if it moves to a different orbit
The Quantum Staircase
The lowest energy orbit is called the ground state
All other energy orbits are called excited states
• An electron:
absorbs a photon if it moves from a lower energy orbit to a higher energy orbit
emits a photon if it moves from a higher energy orbit to a lower energy orbit
Fireworks and Neon Signs
These glow with distinct colors because electrons absorb energy (from heat or electricity) and, when they drop between energy levels, they emit specific wavelengths of visible light
Atomic Spectra
Visible light will refract through a prism and create a continuous spectra of colors – called a rainbow
A sample of a pure gaseous element does not emit a continuous spectra of light when it is exited (energized)
are like an element’s fingerprints – they are unique for each element
Spectral Lines of Atomic Hydrogen
Ultraviolet series n2=1
Visible Light series n2=2
Infrared series n2=3