unit 4: solutions and solubility
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
Solution:
homogeneous mixture
Solute:
substance being dissolved, part of the solution present in the smaller amount
Solvent:
part of the solution present in the greatest amount, does the dissolving
Miscible:
liquids that mix completely
Immiscible:
unable to mix
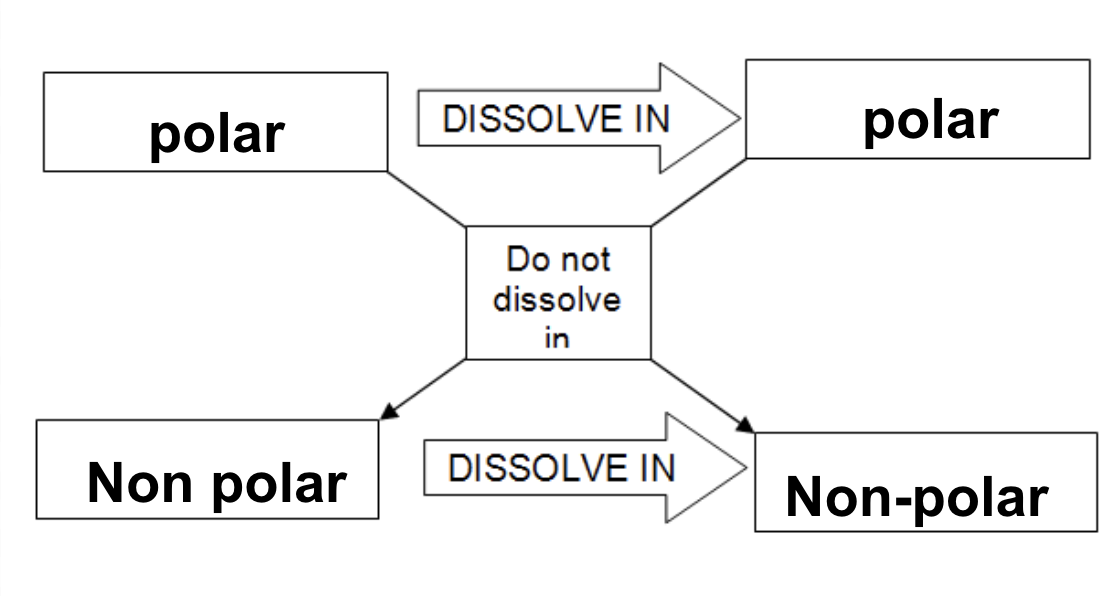
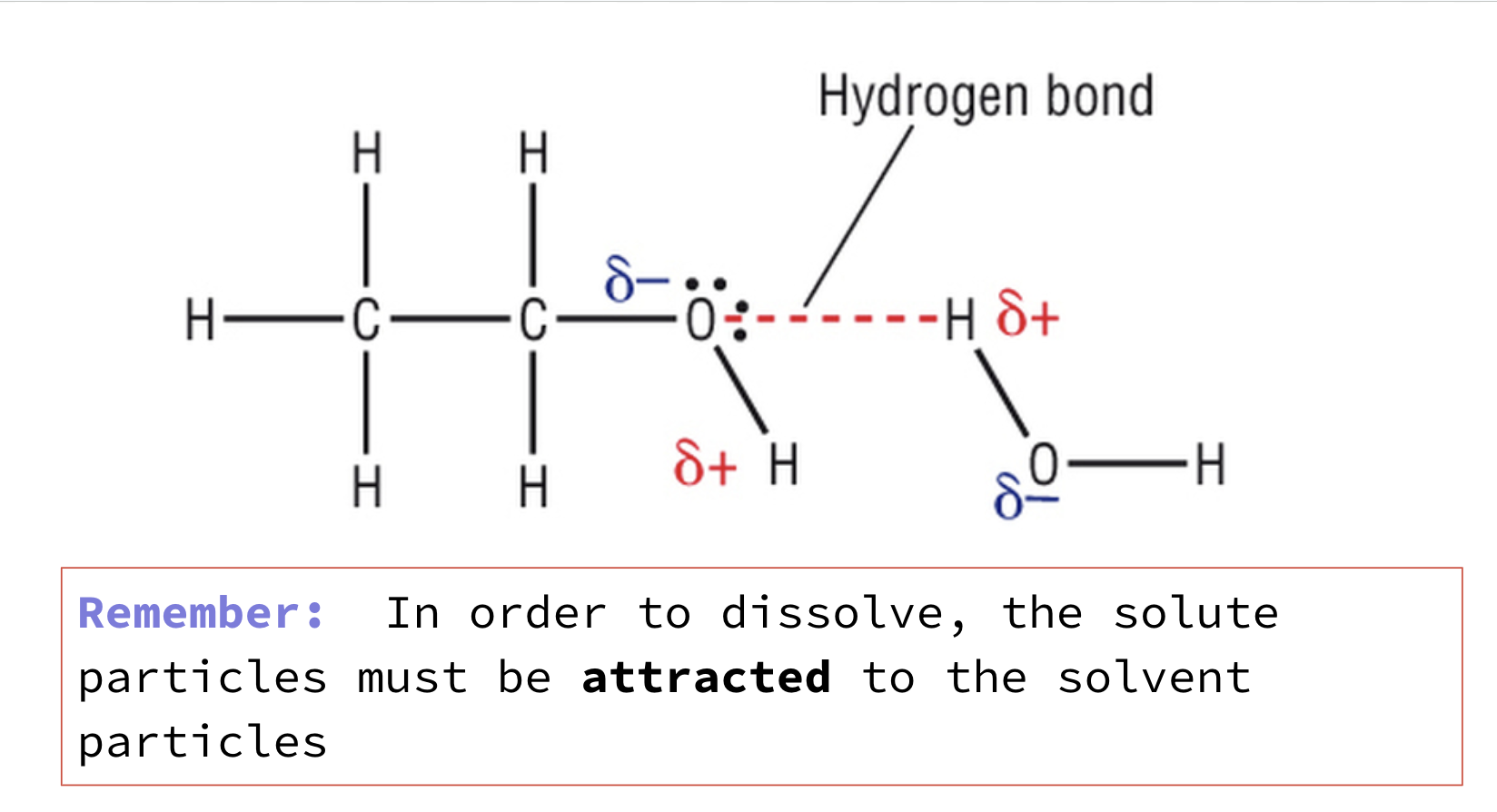
SOLUBILITY AND FORCES BETWEEN PARTICLES
In order to dissolve:
the solute particles must be attracted to the solvent particles
the intermolecular forces between solute and between solvent particles need to be broken
Types of Solute & Solvent Particles
Ionic and polar solutes will dissolve in polar solvents because particles of both are charged
Polar solutes will dissolve in polar solvents
Non-polar solvents dissolve non-polar solutes due to similar intermolecular forces (no full or partial charges)
Recall: ∆EN of non-polar molecules is < 0.5 (0.4 and below), polar molecules is between 1.6-0.5, and ionic compounds is 1.7 and above
This relationship is summarized in the expression:
“Like dissolves like”
“Like dissolves like”
2. Temperature (for solids)
For Solids:
Increases in temperature causes increased solubility as a higher in temperature causes:
Spaces between particles increase resulting in more space for particles of solute to dissolve
Solvent particles have greater kinetic energy which results in more frequent and energetic collisions with the solute
temperature for gases
Increases in temperature causes decreased solubility
Molecules in gaseous state have higher kinetic energy than those dissolved in the solvent
Increasing temperature provides energy for gas molecules to escape solution
3. Pressure
Pressure is force per unit area
No effect on solubility of solids or liquids
Solubility of a gas is directly proportional to the pressure of that gas above the liquid
Increased pressure causes increased solubility of a gas
4. Size
Covalent Compounds
Increased molecule size (molecular compounds) causes decreased solubility
Molecules like methanol (CH3OH) have a non-polar (CH3) end and a polar (OH) end
The –OH group predominates and allows the entire molecule to be soluble in water (polar molecule)
Increasing the size of the non-polar portion decreases solubility
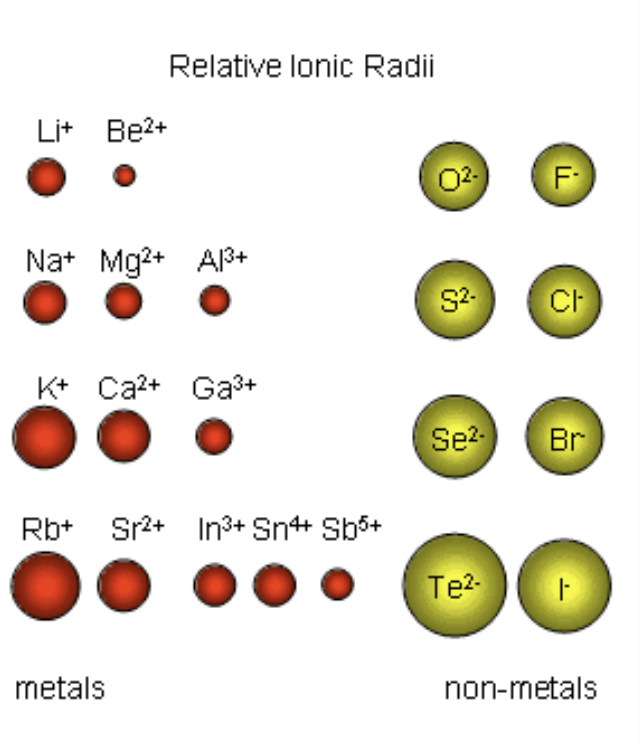
size and ionic compounds
If the attraction between the ions is very strong, they will be difficult to separate, and won’t be soluble in water
Solubility usually increases with increased ion size and decreased ion charge (higher, more concentrated charge, stronger attraction between ions, harder to separate, harder to dissolve)
Factors that affect rate of dissolving
How quickly a solute dissolves in a solvent will increase when each of the following is increased:
Agitation or mixing: increases number of collisions
Temperature: increased kinetic energy causes more frequent collisions
Surface area: more solute is in direct contact with the solvent
Solubility
refers to if a substance dissolves in another (the amount of solute that is able to dissolve in a given quantity of solvent)
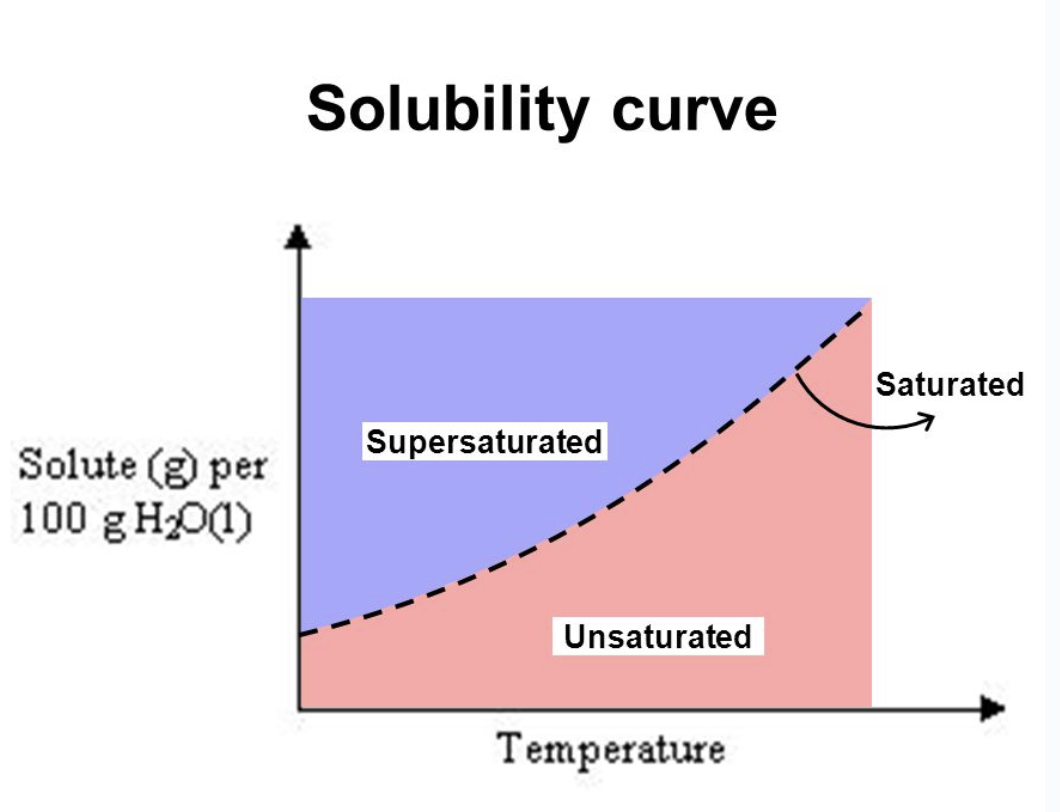
Saturated
the max amount of solute is dissolved at that temperature
Unsaturated
less than the max amount of solute is dissolved at that temperature
Supersaturated
more than the max amount of solute is dissolved at that temperature
solubility curve
Increasing temperature will increase solubility of solids in a solution
A solubility curve shows the relationship between the solubility of the solute and the temperature of the solution.
Solubility curves provide information on the solubility of a substance as we change the temperature of solution.
percent concentration
used for fairly large concentrations
where the solute is a large proportion of the solution
formula: %conc = amount solute/amount solution x 100
3 ways to get percent concentration
% m/m (percent mass/mass) (ex: g and g)
% m/V (percent mass/volume) (usually g/mL)
% v/v (percent volume/volume) (ex: mL and mL)
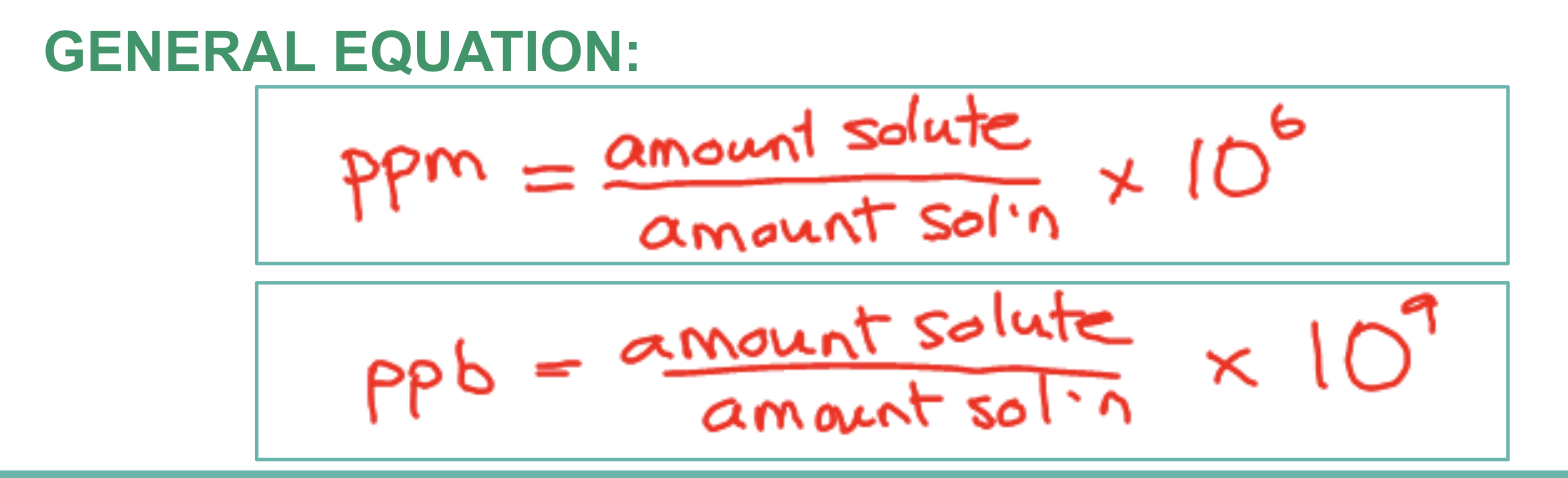
ppm and ppb
These units are used to describe the concentration of substances which are present in VERY SMALL AMOUNTS in solution. UNITS MUST MATCH!
molar concentration
Most useful unit of concentration
Number of moles of solute per litre of solution
Aka Molarity (M)
C = Concentration (mol/L or M)
n = moles (mol)
V = volume (L)
C = n/V
dilutions
When we dilute a solution, we move the solute particles apart
No new solute is added, therefore the number of particles stays the same
The volume changes
Therefore, the concentration changes
dilution sensory clues
When we dilute a solution, there are sensory clues that tell us the particles have spread out. These include:
A lighter colour than the concentrated solution
A weaker odour if the concentrated solution had an odour
dilution equation
General Equation for DILUTIONS ONLY!!
This equation is NOT for stoichiometry questions! Here, the substance stays the same! If the substance changes, you need a mole ratio.
C1V1 = C2V2
C1: concentration solution
C2: diluted solution
properties of ionic compounds
high melting point
hard and brittle
conduct electricity in the dissolved form
soluble in water, insoluble in cyclohexane
disassociation equations
Dissociation equations show the breaking up of compounds into ions (when they dissolve)
ex: Na2SO4 (S) → 2Na+(aq) + SO4 2-(aq)
not all ionic compounds are dissolvable
… not ALL ionic compounds completely dissolve
Some ionic compounds are insoluble (do not dissociate)
The bonds between these ions are strong and are not able to be replaced by water molecules
when are solubility rules used
Used to predict which chemicals will form precipitates (solid) when reacted.
These types of reactions are called precipitation reactions.
write precipitation reactions in 3 steps
balanced chemical equation
total ionic equation
net ionic equation
balanced chem equation
ex: Pb(NO3)2(aq) + Na2CO3(aq) → PbCO3(s) + 2NaNO3(aq)
total ionic equation
Pb2+(aq) + 2NO3-(aq) + 2Na+(aq) + CO32-(aq) → PbCO3(s) + 2Na+(aq) + 2NO3(aq)
net ionic equation
Pb2+(aq) + CO32-(aq) → PbCO3(s)
solution stoich steps
Balance equation
Convert to moles
Mole ratio
Convert from moles to desired quantity (mass, particles etc)
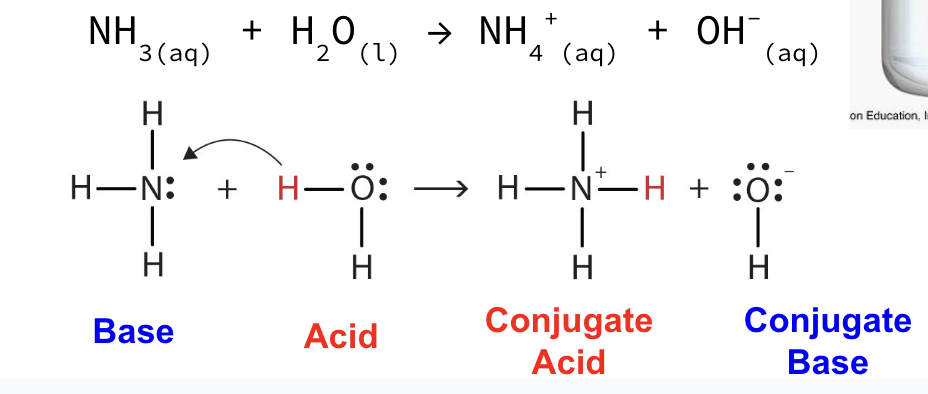
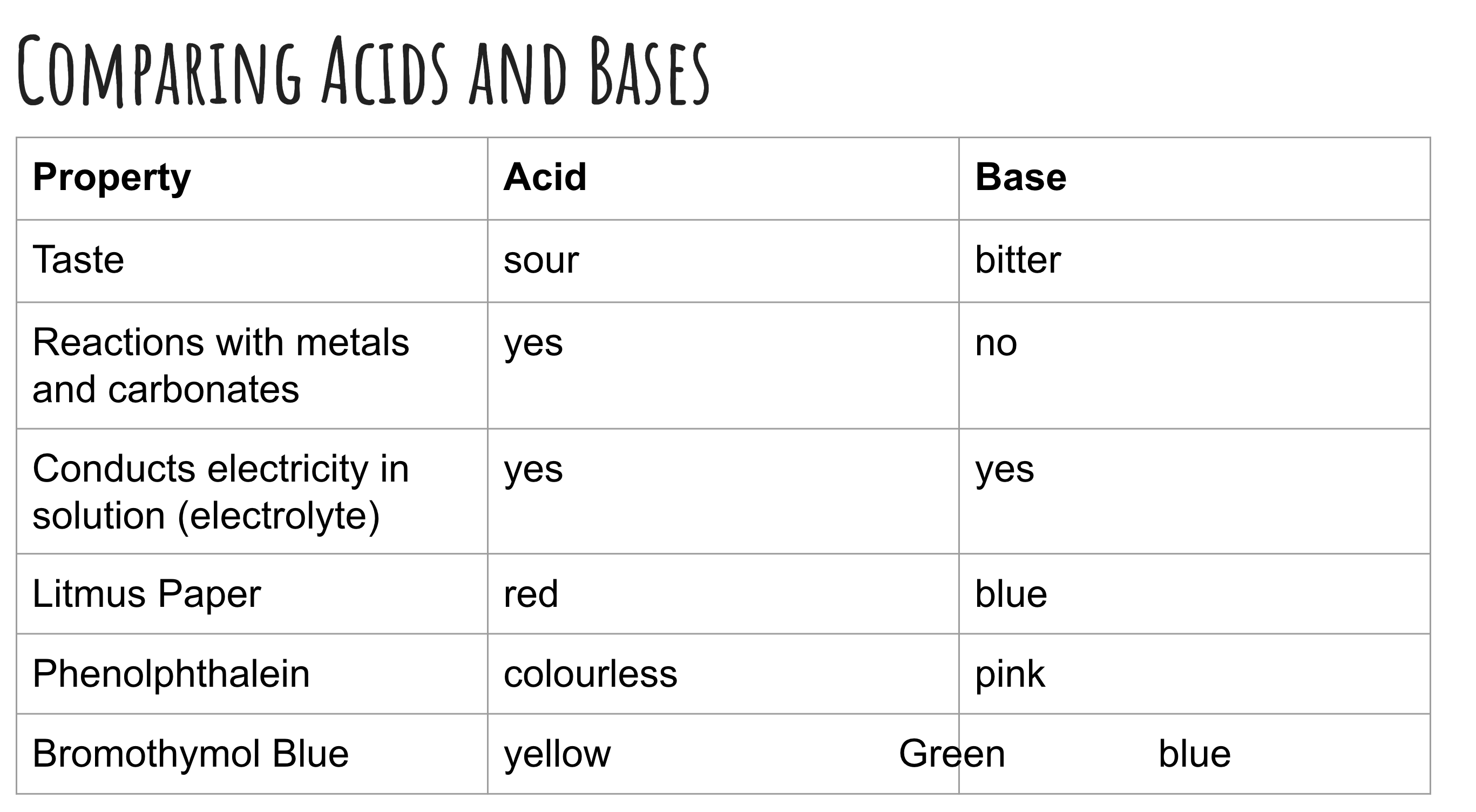
acids
Ionize in solution
Proton donors (Bronsted-Lowry definition)
Produces hydrogen ions, H+(aq) in water according to Arrhenius definition)
The hydrogen ion (H+) bonds with a water molecule (H2O) to make a hydronium ion, H3O+(aq)
strong acids
Ionize extremely well
Completely react with water to make H3O+
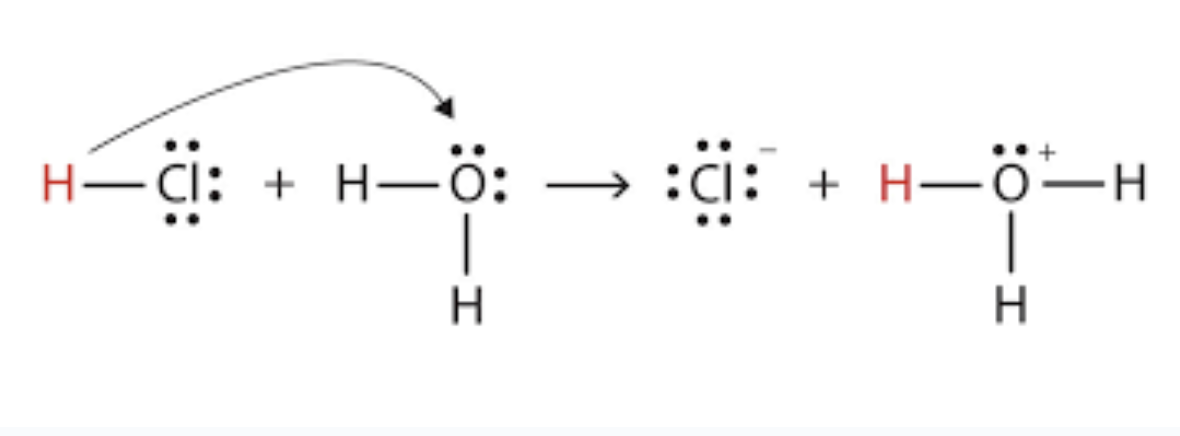
strong acids
Ionize extremely well
Completely react with water to make H3O+
H2SO4(aq) + 2H2O(l) → 2H3O+(aq) + SO42-(aq)
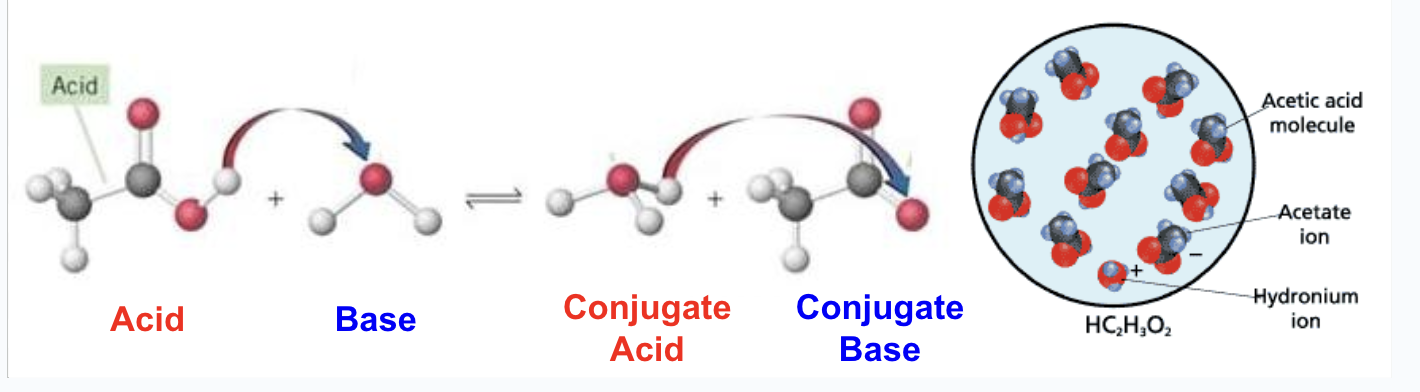
Weak Acids
Only a small percentage ionize(most acids are weak acids)
HC2H3O2(aq) + H2O(l) → H3O+(aq) + C2H3O2-(aq)
Bases
Produces hydroxide ions, OH-(aq) (Arrhenius definition)
Proton acceptors (Bronsted-Lowry definition)
Bitter and slippery
Conducts electricity in solution
Strong bases
Dissociates 100% in aqueous solution
Example
NaOH(s) → Na+(aq) + OH-(aq)
KOH(s) → K+(aq) + OH-(aq)
Weak base
Ionizes poorly
Only a small percentage ionize
comparing acids and bases
pH scale
[H3O+] > [OH-] means concentration of hydronium ions is greater than concentration of hydroxide ions
![<p>[H<sub>3</sub>O<sup>+</sup>] > [OH<sup>-</sup>] means concentration of hydronium ions is greater than concentration of hydroxide ions</p>](https://knowt-user-attachments.s3.amazonaws.com/1e4ab389-a806-4ddb-b687-9bce041bfc49.png)
calculating pH
pH = -log [H3O+]
[ ] means concentration
[H3O+] means conc. of hydronium or H+
calculating pOH
pOH = -log[OH-]
converting pOH to pH
pH + pOH = 14