CHAPTER 2: ATOMIC STRUCTURE AND RADIOISOTOPES CPQ
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Which isotope of Zirconium has the fewest number of neutrons?
| Natural abundance | |
A | 90Zr | 52% |
B | 91Zr | 11% |
C | 92Zr | 17% |
D | 94Zr | 17% |
E | 96Zr | 3% |
Choice A
How many electrons are in the valence energy level?
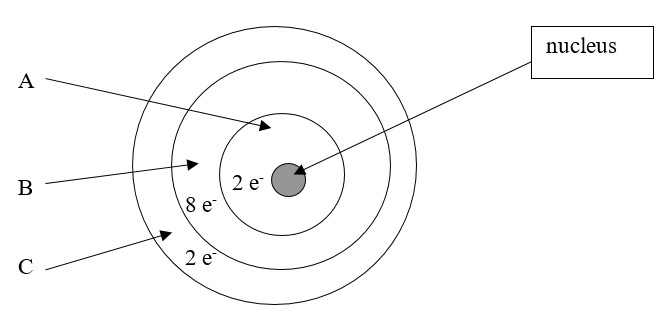
2
Which of the following is NOT the same for different isotopes of the same element?
number of protons
charge
atomic number
number of electrons
mass number
mass number
____ is an important component of the immune system as well as required by many enzymes.
Zinc
According to the current model of the atom, the part of the diagram labeled B is made up of
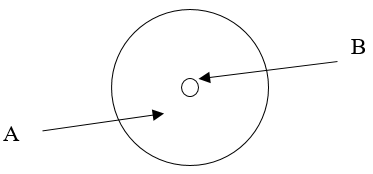
protons and neutrons
____ are neutral.
Neutrons
What is a common symptom of iodine deficiency?
enlarged thyroid
According to the periodic table, what types of elements are in group 1A?
alkali metals
Isotopes are elements with the same number of
protons but different numbers of neutrons.
____ is an important component hemoglobin. Without this protein, tissues become starved of oxygen, and fatigue and shortness of breath results.
Iron
What is the mass number of element X?
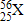
56
According to the periodic table, what types of elements are in group 7A?
halogens
According to the periodic table, how many valence electrons do the elements in the third row have.
It depends on the specific element.
What is the atomic number of element X?
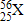
25
Which of the following is NOT true for the atoms 12C, 13C, and 14C?
They all have six electrons.
They all have the same mass number.
They are isotopes.
They all have six protons.
They all have the same atomic number.
They all have the same mass number.
Which of the following elements exists as an isotope with a mass number of 35 and an atomic number of 17?
tellurium
chlorine
bromine
sulfur-35
argon
chlorine
____ have a mass of approximately 1 amu.
Protons and Neutrons.
According to the periodic table, the atomic mass of potassium (K) is ____.
39.10
____ have a negative charge.
Electrons
____ is most commonly ingested along with salt.
Iodine
The mass of an element is determined by:
protons and neutrons
Which pair correctly matches an element to its atomic number?
12.01 – C
9 – F
133 – Cs
39.94 – Ar
9.012 – Be
9 – F
How many electrons are in a neutral atom of element X?
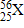
25
Which pair does NOT correctly match an element symbol to its full name?
Cl – chlorineN – nitrogen
H – helium
C – carbon
O – oxygen
H – helium
_____ are the subatomic particles that have the smallest mass.
Electrons
The identity of an element is determined by its number of _____.
Protons
Select the choice in which atomic number, mass number, number of neutrons, and number of protons listed is correct for phosphorous-32.
| Mass number | Number of neutrons | Number of protons | |
a. | 15 | 32 | 32 | 15 |
b. | 15 | 32 | 17 | 15 |
c. | 17 | 15 | 15 | 17 |
d. | 17 | 32 | 32 | 17 |
e. | 16 | 32 | 16 | 16 |
Choice B
According to the periodic table, what types of elements are in group 8A?
noble gases
The number of protons is equal to the ____ in a neutral atom.
number of electrons
According to the current model of the atom, the part of the diagram labeled A is made up of
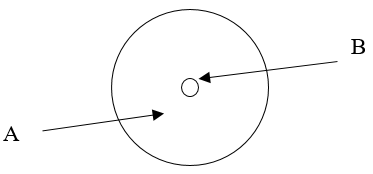
electrons.
Adding ____ to drinking water is a common practice in many cities, meant to strengthen tooth enamel and decrease dental cavities.
flourine
_____ have a positive charge.
Protons
According to the periodic table, what types of elements are in group 2A?
alkaline earth metals
According to the periodic table, the atomic number of potassium (K) is ______.
19