Unit 1 - USCE
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
nucleons
subatomic particles present in the nucleus
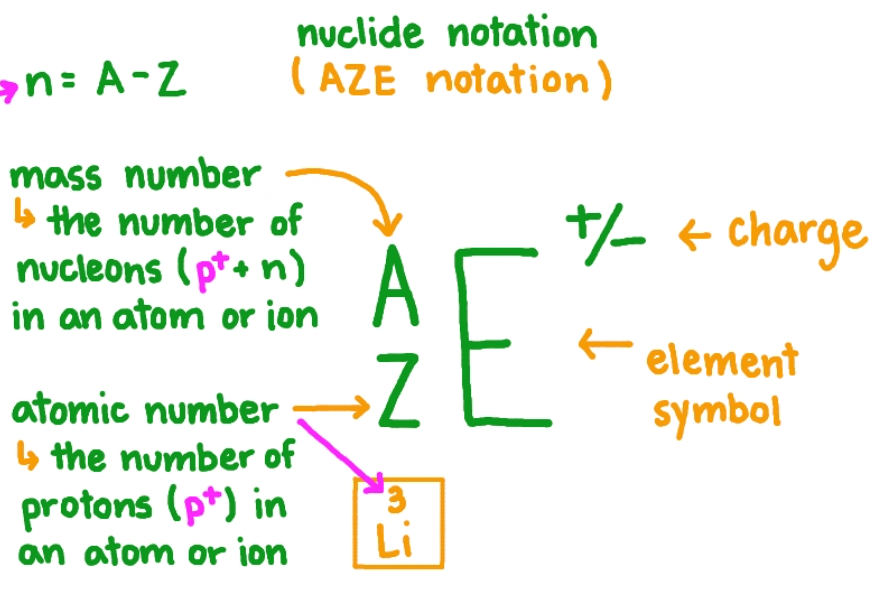
atomic number and mass number
atomic number
number of protons in an element
mass number
sum of number of protons and neutrons in an atom (the mass of electrons is negligible, which is why the nucleons can give the mass of the atom)
atom’s mass in amu (not the same as relative atomic mass)
relative atomic mass
the weighted average of all the naturally occurring isotopes of an element, compared to 1/12th the mass of an atom of carbon-12
what is relative abundance
relative abundance is the percent abundance in nature of a particular isotope
isotopes and radioisotopes
isotope
an atom (not element) with the same number of protons but a different number of neutrons
radioisotopes
isotopes that have ‘unstable’ nuclei emit ‘radiation’ as the nuclei change to become more stable
ground state vs excited state electron
ground state electron
electron in the lowest possible energy level
excited state electron
energised electron that has moved up to a higher energy level
continuous vs line spectrum
In a continuous spectrum all wavelengths of visible light are observed, while in a line spectrum only certain discrete wavelengths of light are observed
atomic orbital
a volume of space where there is a high probability of finding an electron.
what is heisenberg’s uncertainty principle
Heisenberg’s uncertainty principle tells us that we cannot know where an electron is at any given moment; thus electron configurations give us a probability picture of where an electron will likely be.
what is hund’s third rule
Hund’s third rule: If more than one orbital in a sub-level is available, electrons occupy different orbitals with parallel spins.
this means we have to place two electrons in separate orbitals because this configuration minimises their mutual repulsion.
what is the pauli exclusion principle
The Pauli exclusion principle states that no more than two electrons can occupy any one orbital, and if two electrons are in the same orbital, they must spin in opposite directions.
Spin is an important factor in electron-electron interactions, as it allows two electrons to occupy the same region of space despite their mutual repulsion if they spin in opposite directions.
what is the aufbau principle
The Aufbau principle states that electrons are placed into orbitals of lowest energy first, boxes can be used the represent the atomic orbitals. each main energy level (n) is divided into sub-levels, we can find the electron configuration of an atom of an element by using this principle.
what is filtration
use filter paper and a funnel to filter solutes from solvents
used to filter out dirt from water (stuff left in filter paper is called residue)
used for mechanical mixtures of solid and liquid
what is solvation
using a solvent to dissolve the soluble particles, so it leaves the insoluble particles
two solids together (also mixtures)
what is a solute
the minor component in a solution, dissolved in the solvent
thing being dissolved
what is a solvent
thing dissolving the solute
what is distillation
have a mixture (homogenous) two different substances have two different boiling points. (water and rubbing alcohol have different boiling points)
one of them boils, the other one doesn’t, separate them by one of them boiling and the other condensing (travels down condensing tube and separates)
what is evaporation
boiling (separating salt from water, boiling water will cause it to evaporate and leave the salt)
what is recrystallisation
dissolves impure solid mixture into hot solvent, then allow it to cool and crystallise. Using filtration to separate impurities from crystal
a solvent with low solubility at room temperature, and high solubility at high temperatures is used. solubility of a solvent increases at higher temperatures
AZE or nuclide notation
describe the findings of Rutherford’s gold foil experiment
Observations from the experiment:
Some positive alpha particles did pass straight through, but many were deflected by various degrees.
There must be a concentration of positive charge in the atom. The idea of a nuclear atom was developed.
Only a positively charged and relatively heavy target particle, such as the proposed nucleus, could account for such strong repulsion; thus, establishing that protons are not evenly distributed throughout an atom, and that an atom is mostly empty space.
Rutherford had all the electrons moving around the nucleus (like planets around the sun), but they all had the same energy.
Rutherford’s model neglected to explain what prevented the electrons from spiralling into the nucleus of the atom
do isotopes of an atom have the same physical and chemical properties
physical properties such as density and boiling point are slightly different, same chemical properties
why doesn’t relative atomic mass have a unit
because it is measured as a comparison to 1/12th the mass of carbon-12, not in a unit
Copper is made of two naturally occurring isotopes – copper-63 and copper-65. The relative atomic mass of copper can be found on a Periodic Table. Without doing any calculations, which isotope is more abundant? Explain your answer.
From the PT, the relative atomic mass of copper is 63.55. There are two common isotopes of copper – 63Cu and 65Cu. Since the relative atomic mass is closer to 63 than 65, the 63Cu isotope is more abundant than 65Cu.
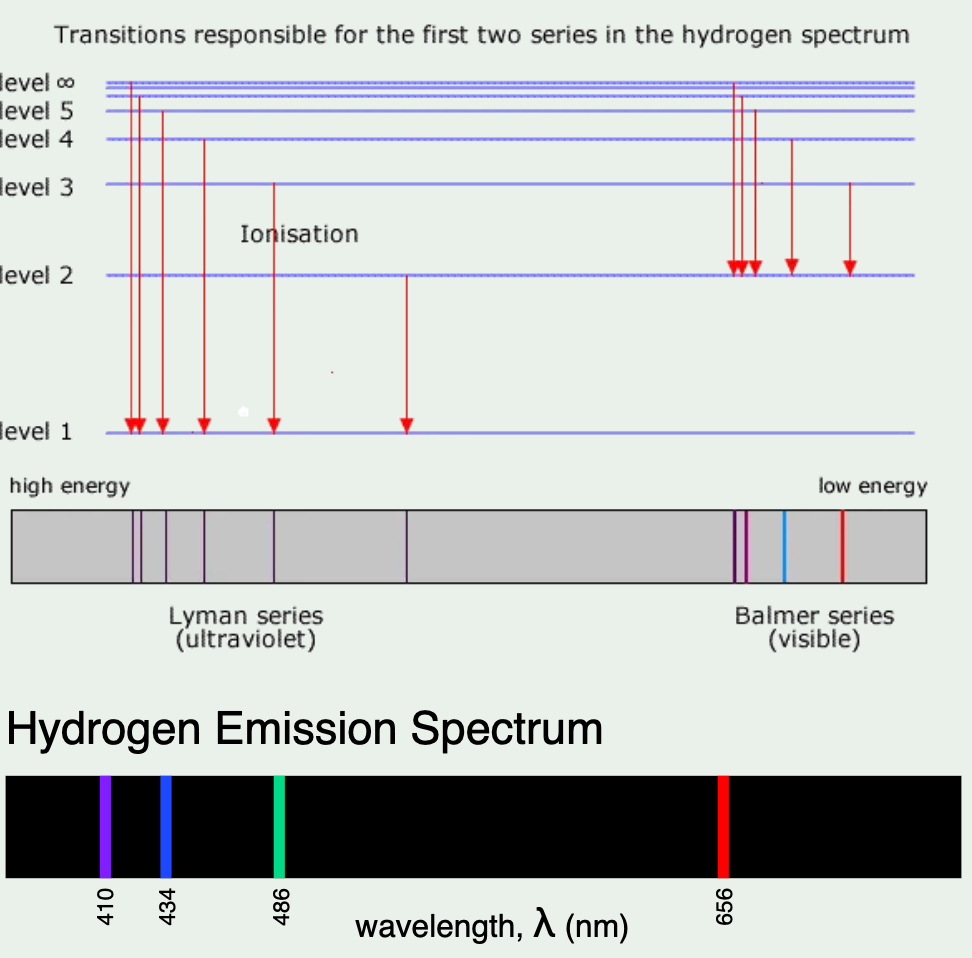
draw the electron transitions for the visible emission spectrum of hydrogen, and describe the emission spectrum of hydrogen
the H emission spectrum illustrates specific wavelengths of colours in the form of a line
the lines in the spectrum converge at higher energies/frequency
outline how the emission spectrum of hydrogen is related to the energy levels in the hydrogen atom?
each line colour produced in H emission emitted by a electron transition from a higher to lower energy level
describe how the visible light line spectrum is produced in a hydrogen atom
Electromagnetic radiation is passed through hydrogen and is absorbed and excites the electrons from a lower energy level to a higher energy level. This is unstable so the electron falls back to the ground state, and releases a photon. The emission spectrum produced is a line spectrum rather than a continuous spectrum because an electron can only change its energy by discrete amounts.
The electron transitions that produce the line spectrum are n=3 → n = 2, n = 4 → n = 2, n=5 → n = 2, and n = 6 → n = 2
explain how the formation of lines indicates the presences of energy levels
Lines indicate that only specific defined energies are released when excited electrons fall to lower levels.
This suggests that electrons can only have certain defined energy values. This supports the idea that electrons may only occupy defined energy levels with specific energy values.
If electrons could exist anywhere outside the nucleus, we would expect a continuous spectrum when excited electrons lose their energy.
Outline the three ranges of the electromagnetic spectrum that electron transitions produce, and their corresponding transition
n=5 → n=3
infrared range (paschen series)
n=3 → n=2
visible light range (Balmer series)
n=6 → n =1
ultraviolet range (Lyman series)
why does the emission spectrum of hydrogen converge at higher frequencies
This is because energy levels get closer together as they get further from the nucleus.
Therefore, the emission spectrum converges at higher frequencies, because as they get closer and closer together, the difference in energy needed to move up to a higher energy level is lower.
Energy released by an excited state electron falling to a lower level is proportional how much they moved up, thus, making the lines in the emission spectrum closer together at higher frequencies.
when calcium compounds are introduced into a gas flame a red colour is seen. sodium compounds give a yellow flame. outline the source of the colours and why they are different
each colour is produced by electron transitions, where an excited state electron fell down to a lower energy level.
They produce different coloured flames due to the different nuclear charge, leading to differing energy difference between energy levels. so, the amount of energy needed to jump to next energy level is different among elements
Helium is usually shown at the top of Group 18 of the periodic table. Give reasons for this position and comment on how it is also misleading.
Helium has 2 electrons in n=1.
It is in period 1 because it has one occupied main electron level. Energy level 1 holds only two electrons, so the valence shells of helium is full. Elements in Group 18 have complete valence shells so He is often seen at the top of Group 18.
The position at the top of Group 18 might be misleading because Group 18 is in the p block. Helium atoms do not have electrons in a p sublevel.
Which electron configurations are exceptions to the rules?
Period 4: Cr, Cu
Period 5: Mo and Ag
Why must the 3d sublevel be filled before 4p
According to Aufbau’s principle, electrons must fill the orbital of the lowest energy first; thus, the 3d sublevel must be filled before 4p
How does losing electrons affect the electron configuration of transition metals
When transition metals become cations, they loose electrons in the outermost energy level first. So Fe would loose atoms from the 4s sublevel before the 3d sublevel