Arterial Blood Gases (ABG)
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
what is an arterial blood gas? what does it measure?
this is an invasive blood test that measures:
- acidity (pH)
- oxygen level (PaO2)
- Carbon Dioxide level (PaCO2)
- Oxyhemoglobin saturation (SaO2)
- Bicarbonate Concentration (HCO3-)
where is an ABG usually done?
- radial, brachial, axillary, or femoral arteries
why do we care about ABGs?
- they monitor patients conditions
- they form the best basis for clinical decision making
- give guidance for interventions like positioning, percussion, ambulation, exercise, and emphasis on oxygenation and deep breathing
- they quantify patients response to therapeutic interventions
- they give an objective overview of patients medical status like if they are ready for therapy or need to be on hold
- gives insight into potential limitations a patient can have while working with PT
when are ABG values needed?
- within 30 minutes of intubation
- anytime there is a question about the patients ventilation, acid-base status, or oxygenation status
what is FiO2?
fraction of inspired oxygen (0.21 is room air)
what is PaO2?
partial pressure of oxygen in arteries
what is PAO2?
partial pressure of oxygen in the alveoli
what is SaO2?
oxyhemoglobin (in arteries, looks to see how well hemoglobin is transporting oxygen)
what is SpO2?
oxygen saturation in the periphery
what is SaCO2?
carbaminohemoglobin (looking to see how much CO2 is bound to hemoglobin)
what is PaCo2?
partial pressure of carbon dioxide in arteries
what is PvO2?
mixed venous oxygen tension
what is HCO3-?
bicarbonate
what is the normal value for room air?
21% or 155mmHg
what is the normal value for PAO2?
100mmHg
what is the normal value for PaO2?
80-100mmHg
what is the normal value for PvO2?
40mmHg
what is the alveolar - arterial pressure gradient estimation?
(age + 10)/4
what is the actual alveolar arterial pressure gradient (how is it calculated)?
PAO2 - PaO2
what is the term for decreased oxygen in tissues?
hypoxia
what is the term for decreased oxygen in the blood?
hypoxemia
what value indicates hypoxemia?
PaO2 <80mmHg
what are some causes of hypoxemia?
- decreased oxygen content of inspired air (altitude)
- hypoventilation
- shunting
- diffusion deficits (a-c membrane interface)
- ventilation perfusion deficits (V/Q mismatch)
what are some signs/symptoms of hypoxemia?
- cyanosis
- tachypnea
- arrhythmias
- diaphoresis
- confusion
- restlessness
- poor judgement
- change in motor coordination, slower reaction times
- dizziness
- headache
how is oxygen transported in the blood?
its transported by hemoglobin molecules (oxyhemoglobin) and dissolved in the blood as PaO2
1 hemoglobin can bind how many oxygens?
4
what does the oxygen dissociation curve tell us?
how well oxygen is binding to hemoglobin
(so hemoglobin concentration and PaO2 levels)
what does it mean when the oxygen dissociation curve shifts right?
release of oxygen from hemoglobin to tissues (so less oxygen is bound to hemoglobin)
why would the oxygen dissociation curve shift right?
- increased H+ (acidosis)
- increased temperature
- increased altitude
- exercise
- increased 2-3 BPG
in summary - hemoglobin has a low affinity to bind to oxygen and the tissues are taking in as much oxygen as they can rapidly
what does it mean when the oxygen dissociation curve shifts left?
- more oxygen is bound to hemoglobin (so not being offloaded)
why would the oxygen dissociation curve shift left?
- decreased H+ (alkalosis)
- decreased temperature
- decreased altitude
- decreased 2-3 BPG
Summary: tissues dont have as much oxygen (and dont need it!)
if the H+ ions are high in number, would the pH be high or low?
low
if the H+ ions are low in number, would the pH be high or low?
high
what is an acid?
a substance that can yield or donate a hydrogen ion when dissolved in water
what are some systems that produce a lot of acid?
- anaerobic metabolism
- CO2 production
- fatty acid oxidation
- ketone production
- phospholipid metabolism
what is a base?
this is a substance that combines or accepts hydrogen ions creating an excess of hydroxide ions (OH-)
so they gobble up H+ and leave OH- behind (which increases the pH)
do we produce a lot of bases in the human body?
nope
what is a buffer solution?
this is a solution that has the ability to resist changes in pH upon the addition of small amounts of either acid or base
what does a buffer solution contain?
- a weak acid or base AND a conjugate acid or conjugate base
when is a buffer solution most effective?
when the proton donor (a weak acid) and proton acceptor (weak base) are in equal concentrations
what are some common buffers in the body?
- protein
- hemoglobin
- phosphate
- ammonia/ammonium ion
- carbonic acid/bicarbonate *** (big dawg)
most cells in the body constantly generate what?
carbon dioxide
what happens with the carbon dioxide once our bodies produce it?
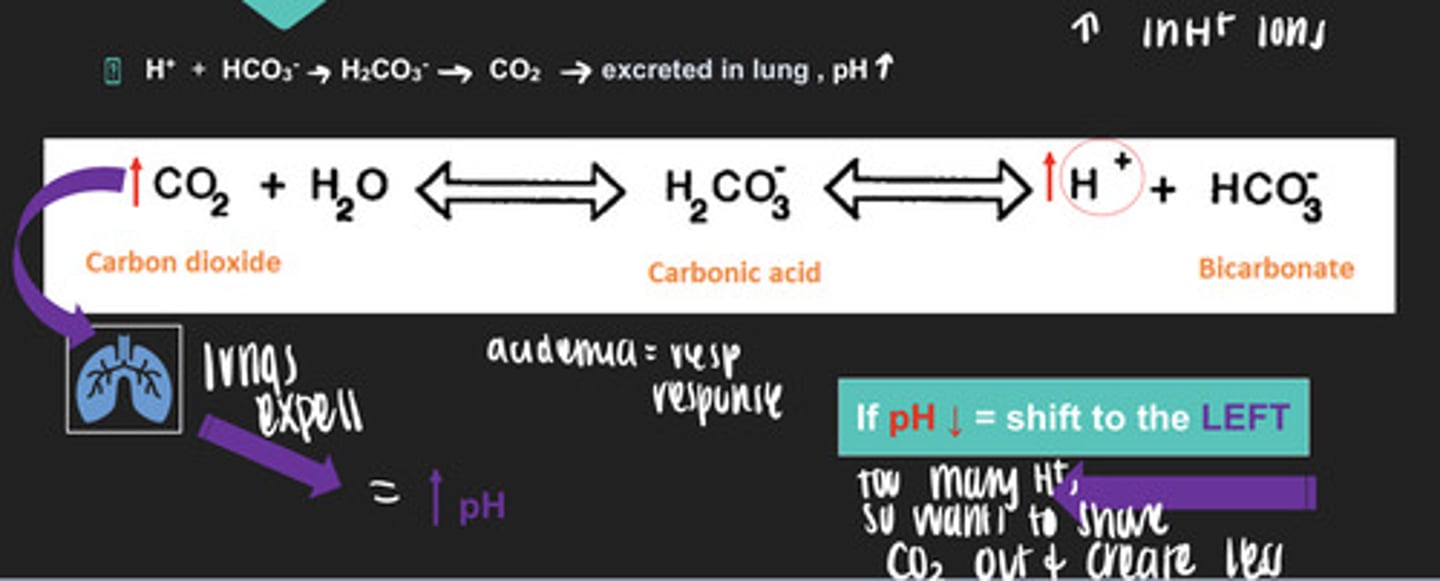
it binds to water to form carbonic acid which then dissociates into H+ and bicarbonate
how does the carbonic acid/bicarbonate buffer system respond to a decreased pH?
- the equation shifts to the left!
so you have too much H+ and you want to shove out the carbon dioxide and create less... sooo you use your lungs to expel the carbon dioxide out which increases the pH
how does the carbonic acid/bicarbonate buffer system respond to an increased pH?
- the equation shifts to the right!
you have too much HCO3-, so you want to expel the base via the kidneys to urine so that pH goes down.
What does -emia mean?
a state of being in the blood
what does -osis mean?
a process that is occurring
what is acidosis?
a process making pH lower
what is alkalosis?
a process that makes pH higher
what type of disorder is related to the HCO3- concentration and is controlled by the kidneys?
metabolic disorders
what type of disorder is related to PCO2 concentration and is controlled by the lungs?
respiratory disorders
what is compensation?
a method the body uses to normalize pH
what type of disorder do these characteristics describe?
- pH decrease
- HCO3- decrease
- H+ increase
metabolic acidosis
what causes metabolic acidosis?
- diabetic ketoacidosis
- renal failure (kidneys fail to get rid of the garbage)
- diarrhea
what type of disorder do these characteristics describe?
- pH increase
- HCO3- increase
- H+ decrease
metabolic alkalosis
what causes metabolic alkalosis?
- vomiting (great loss of electrolytes)
- Iatrogenic (can just happen)
what type of disorder do these characteristics describe?
- pH decreases
- PaCO2 increases
respiratory acidosis
what causes respiratory acidosis?
hypoventilation (decreased RR)
what can cause hypoventilation?
- CNS depressant
- neuromuscular disorders
- Obstructive lung disease
what type of disorder do these characteristics describe?
- pH increases
- PaCO2 decreases
respiratory alkalosis
what causes respiratory alkalosis?
hyperventilation
what causes hyperventilation?
- CNS hyperactivity (stress, fear, pain, sepsis, anxiety)
- hypoxia
primary respiratory disorders have what type of compensation?
metabolic
primary metabolic disorders have what type of compensation?
respiratory
if you have a disorder of respiratory acidosis, causing decreased pH and PaCO2 to be increased, how would your body compensate?
you would want to increase HCO3- (metabolic compensation)
if you have a disorder of respiratory alkalosis, causing increased pH and PaCO2 to be decreased, how would your body compensate?
you would want to decrease HCO3- (metabolic compensation)
if you have a disorder of metabolic acidosis, causing decreased pH and decreased HCO3-, how would your body compensate?
decrease PaCO2 (respiratory compensation)
if you have a disorder of metabolic alkalosis, causing increased pH and increased HCO3-, how would your body compensate?
increase PaCO2 (respiratory compensation)
how do we do respiratory regulation of pH?
- change respiratory rate and depth ventilation
what senses the change in the body's carbon dioxide and pH levels?
chemoreceptors
once chemoreceptors sense a change they do what?
alert the respiratory center in the brainstem to either decrease RR and depth ventilation, or increase RR and depth ventilation
how long does it take for respiratory regulation of pH?
minutes to hours
chemoreceptors will become what if you have chronic elevated levels of CO2?
insensitive
if your H+ increased and CO2 increased what would happen to your RR and depth ventilation?
it would increase RR and depth ventilation to increase pH
if your H+ decreased and CO2 decreased what would happen to your RR and depth ventilation?
decrease RR and depth ventilation to decrease pH
how do the kidneys regulate pH?
- excreting H+ in the urine
- reabsorbing HCO3- in the proximal tubules
- increased production of HCO3- in the collecting tubules
how long does it take for the kidneys to regulate pH?
within hours to days
what is your bodys response to acidemia?
- your lungs increase RR
- your kidneys secrete H+ through urine
what is your bodys response to alkalemia?
- decreased RR
- secretion of HCO3- through urine
what is normal pH level?
7.35-7.45
what is normal paO2 level?
80-100mmHg
what is normal PaCO2 level?
35-45 mmHg
what is normal HCO3- level?
22-26 mEq/L
what are the 5 steps to interpreting ABGs?
1. Assess pH
2. Assess Respiratory Component
3 Assess Metabolic Component
4. Determine Primary Disturbance
5. Assess for Compensation
what is something to note with acidosis or alkalosis if assessing pH?
you can have acidosis or alkalosis even if the pH is in normal range due to body compensation
what value gives us information about whether there is compensation occurring for the primary disturbance?
HCO3-
how do we determine the primary disturbance?
look at PaCO2 in relation to pH
if there is an inverse relationship between PaCO2 and pH what is the primary disorder?
likely respiratory
if there is NO inverse relationship between PaCO2 and pH the primary disorder is likely what?
metabolic
how can we assess for compensation?
look at the PaCO2 values and HCO3- values in the context of the primary disturbance
if you have a primary respiratory disorder you likely have what compensation?
metabolic compensation
(looking at HCO3- levels... right???)
if you have a primary metabolic disorder, you likely have what type of compensation?
respiratory compensation (looking at PaCo2 levels... right?)