Phagocytosis + phagosome maturation
1/37
Earn XP
Description and Tags
define process of phagocytosis, explain how phagocytosis and phagosome maturation occurs, discuss variability of phagosome maturation and how some pathogens escape phagocytosis
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
what are the 2 functions of phagocytosis?
killing pathogens and allowing for tissue homeostasis/remodelling by getting rid of dead cells
summarise the main steps of phagocytosis
target recognition, engulfment, destruction of pathogen, antigen presentation and waste disposal
what happens during phagosome maturation?
its pH becomes more acidic and fusion with a lysosome introduces more enzymes to break down the pathogen
what are the two ways phagocytic targets can be recognised?
direct, recognising the pathogen surface, indirect - recognising the pathogen through complement proteins or antibodies
what category of receptors is responsible for recognising pathogens directly?
pattern recognition receptors
as well as DNA and peptidoglycan, what are some other PAMPs of gram-positive bacteria?
lipoteichoic acid and lipoproteins (both cell wall components)
what are 3 PAMPs that phagocytes recognise on fungi?
beta-glycan, mannan, zymosan
why do protozoa and fungi have less PAMPs than viruses and bacteria?
they are eukaryotic and contain more surface proteins similar to humans
name 3 main PRRs in phagocytes
mannose receptor, scavenger receptor, dectin-1 (glucan) receptor
when is the mannose receptor dominant in initiating phagocytosis?
during pneumocystis
which family of receptors is the mannose receptor part of?
C-type (calcium dependent) lectin
other than phagocytes, which other innate immune cells express scavenger receptors?
natural killer cells - to destroy abnormal host cells
what 2 PAMPs can SR-A recognise?
LPS or lipoteichoic acid
which PRR is primarily responsible for the engulfing of fungi?
dctin-1
what does dectin-1 receptor recognise?
beta-1,3-glucan
what are complement proteins and antibodies both examples of?
opsonins
which immunoglobulin family mainly triggers phagocytosis?
IgG
which part of the antibody do phagocytes recognise?
Fc
what is the IgG receptor on phagocytes known as?
FcgammaR1
give an example of a complement receptor found on phagocytes, and its ligand
CR3 (heterodimer complex of CD11b + CD18), iC3b (fragment of C3b)
what drives phagocytosis?
actin polymerisation allows for membrane extension, fusion of membranes to create vesicles and particle retraction to take up the pathogen
how is Fc receptor-mediated phagocytosis initiated?
upon recognition of a multivalent IgG-labelled pathogen, FcγRs will cluster when binding to the IgG’s Fc portion. this binding will lead to phosphorylation of Fc receptor ITAMs to start signal
what happens downstream of ITAM phosphorylation of Fc receptors at the beginning of phagocytosis?
phosphorylated ITAMs will recruit Syk that activates CrkII and allows for recruitment of Dock180. Dock180 is a guanine exchange factor that activates GTPases which are needed for the activation of actin nucleating complexes e.g. Scar WAVE to initiate actin polymerization via Arp2/3
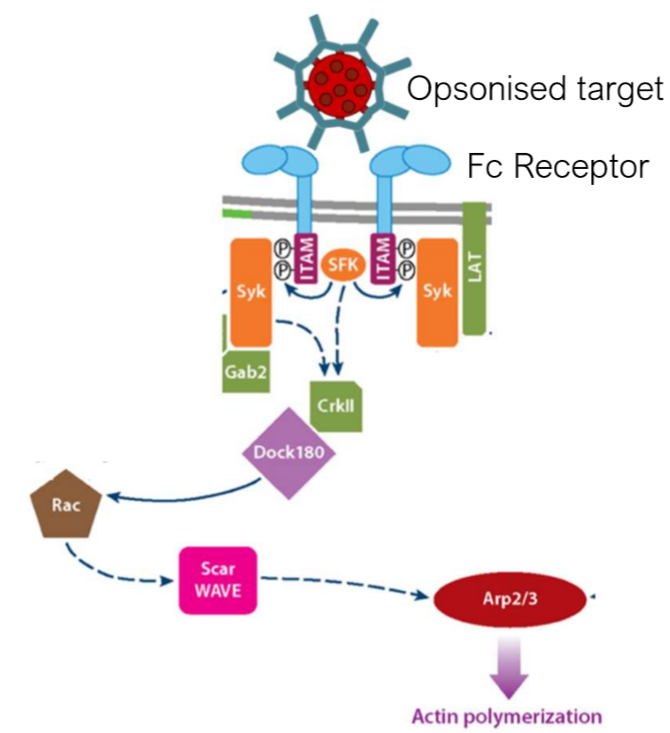
why are vesicles crucial for phagocytosis?
if the pathogen is broken down freely in the cytoplasm, the rest of the cell would also be digested
what kind of budding events occur in phagocytosis?
when enzymes required to digest the pathogen are packaged in the Golgi and bud off (as endosomes!) to be transported to the phagosome e.g. vacuolar ATPases for acidification
how is the pathogen engulfed?
the cytoskeleton is disrupted by F-actin debranching and severing proteins. new actin filaments are nucleated by Arp2/3 to form pseudopodia. GTPases (Cdc42) activate N-WASP to activate Arp2/3 at the phagocytic cup. to close the vesicle, actin polymerization is terminated and F-actin is removed from the phagocytic cup by PI3K
what happens in early phagosome maturation?
following closure of the phagosome, the surface charge decreases to recruit Rab5 GTPase. this recruits Vps34 for generation of phosphatidylinositol-3-phosphates (PI3Ps) that are signalling lipids to recruit EEA1 to aid early endosome fusion required for increasing acidity of the phagosome
what happens in the early to late transition in phagosome maturation?
Rab5 attracts Mon1 and CcZ1 to recruit Rab7 needed for lysosome accumulation in later stages of maturation. endosomes also pinch off the phagosome to recycle components
what happens in the late phagosome?
Rab7 recruits RILP and ORP1L needed to bind the phagosome to dynein that transports the phagosome to the negatively charged end of microtubules where lysosomes are located (which are also coated in Rab7/RILP/dynein)
what are the 3 phases of phagosome maturation?
early, late, phagolysosome
what makes phagolysosomes very acidic?
the presence of many V-ATPases on their membranes that transport protons into the phagolysosome, lowering the pH. also NADPH oxidase complexes produce negatively charged ROS
how does phagosome maturation differ between FcR-mediated and CR-mediated phagocytosis?
CR-mediated phagocytosis involves weaker ROS bursts
which protein often labels apoptotic cells for phagocytosis?
LC3
how else can phagosome maturation differ?
polarisation of macrophages can change speed of maturation and presence of ROS, ageing also affects macrophage function
how can pathogens evade direct recognition?
encapsulation of PAMPs makes it harder for phagocytes to recognise the pathogen, layering of alpha glucan in fungi which is less well recognised hides beta glucan from dectin-1 receptors
how does mimicking GAPs block phagocytosis?
since GAPs inhibit GTPases, this means that proteins such as Rho and Cdc42 can be blocked, preventing actin polymerisation for engulfment
how can pathogens evade phagosome maturation?
LPG glycolipids can block fusion of late endosomes and lysosomes, presence of lipids can pause maturation
how is opsonisation evaded?
C3b can be scavenged by SdrE in S. aureus, protein A can bind the Fc portion of IgG