boyle's law
0.0(0)
0.0(0)
Card Sorting
1/3
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
1
New cards
what is the boyle's law?
at a constant temperature, the pressure is inversely proportional to the volume of gas. The higher the pressure, the smaller the volume.
2
New cards
Formula?
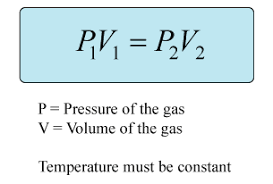
3
New cards
Pressure measurement
1 atm = 100kPa
1 atm = 760 mm-Hg
1 atm = 76 cm-Hg
1 atm = 1 bar
1 atm = 760 mm-Hg
1 atm = 76 cm-Hg
1 atm = 1 bar
4
New cards
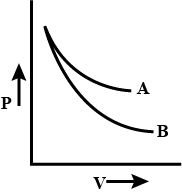
Graph?
The curve is called the isothermal curve. y = k/x. P ~ 1/V.