CHEM EQUILIBRIA
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Define “reversible reaction”.
A reversible reaction is a reaction where the reactants form products that can react with each other to re-form the reactants.
Define “dynamic equilibrium”.
Dynamic equilibrium refers to a reversible process at equilibrium in which the rate of the forward reaction equals to the rate of the backward reaction and the concentrations of reactants and products do not change.
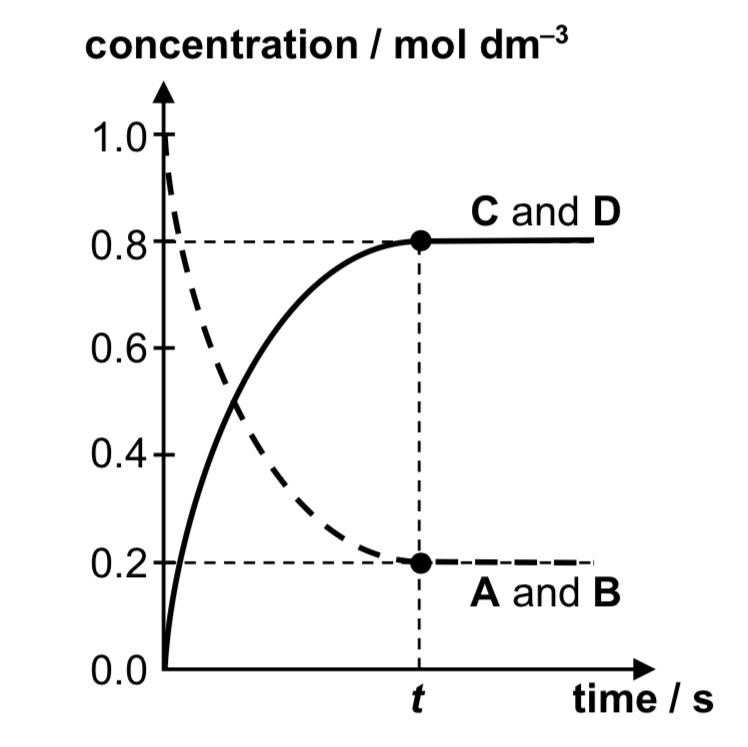
concentration-time graph for A + B ⇌ C + D
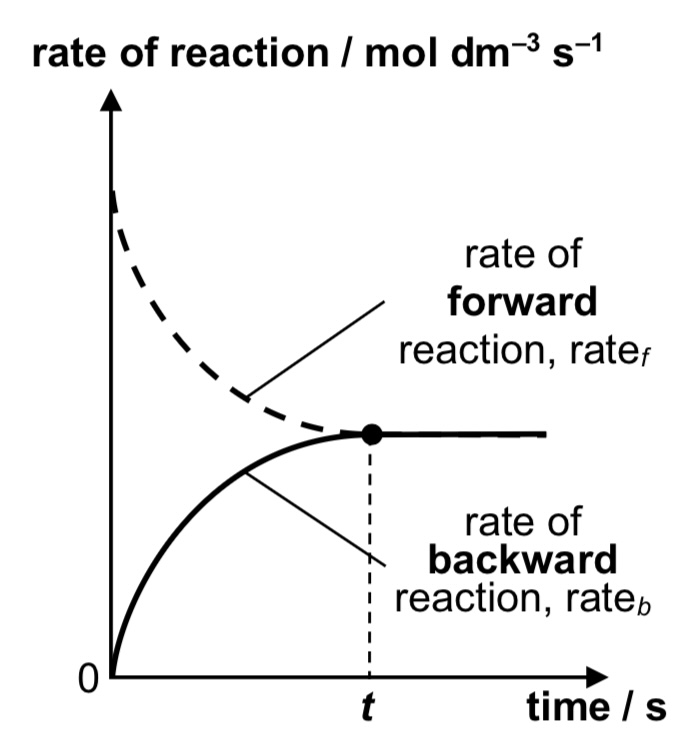
rate of reaction-time graph for A + B ⇌ C + D
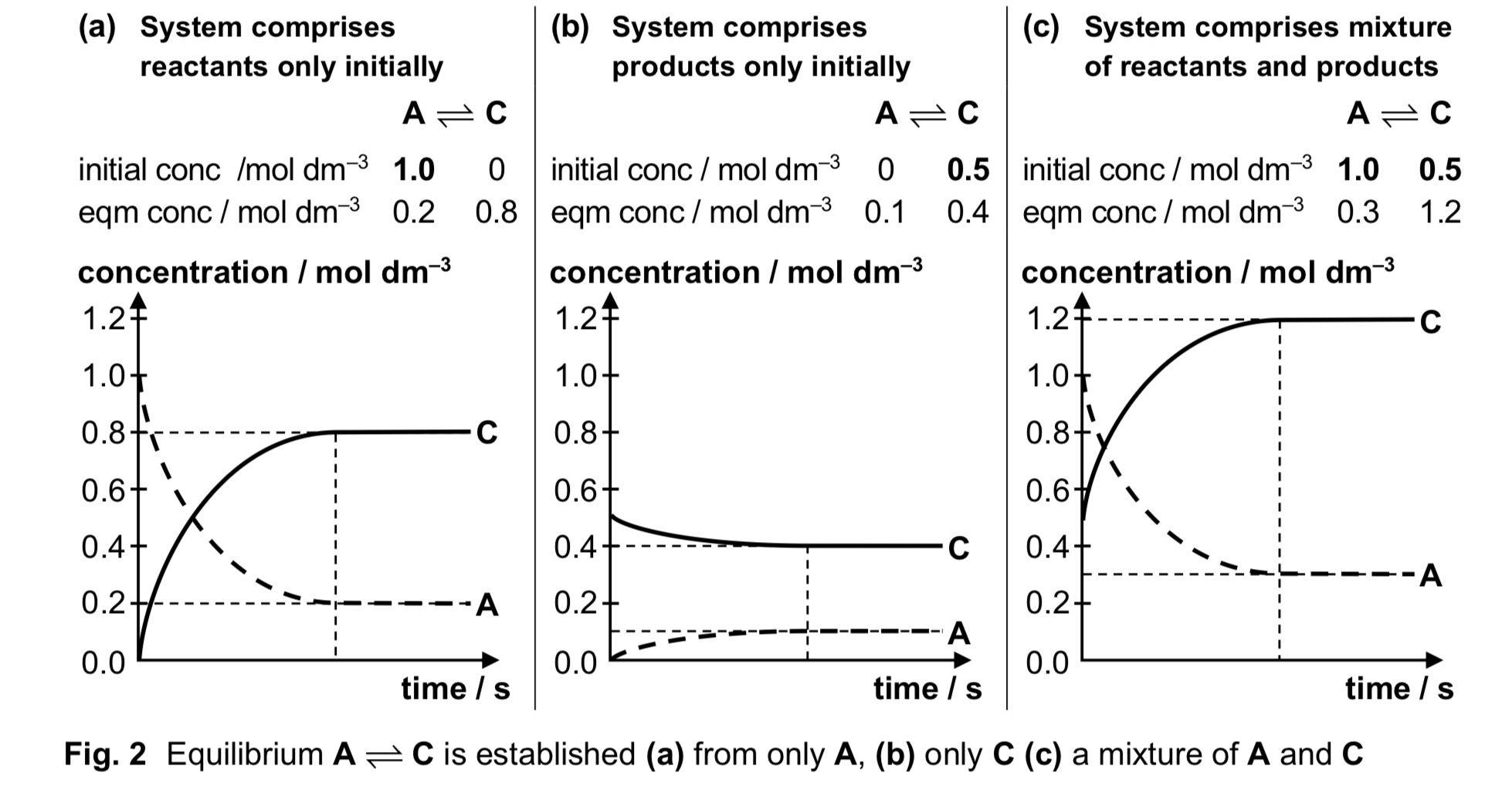
What are the characteristics of a state of dynamic equilibrium?
1) Concentration of all reactants and products remain constant and an equilibrium mixture is obtained
2) Rate of forward reaction is equal to the rate of backward reaction
3) Equilibrium can only be achieved in a closed system, where there is no loss or gain of substances to and from the surroundings
4) Equilibrium can be established from either direction → reactants only, products only, or mixture of reactants and products
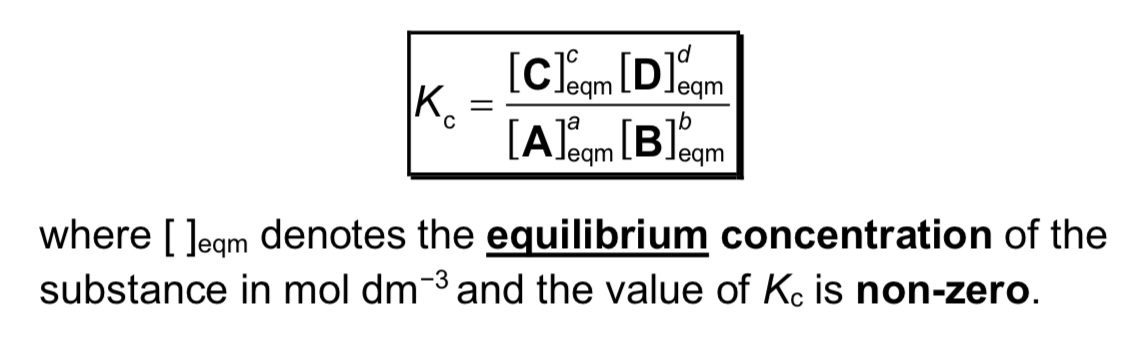
What is Kc, what are its units and when does it change?
→ Kc is equilibrium constant of A+B ⇌ C+D
→ units are (moldm^-3)^c+d-a-b or (moldm^-3)^c+d-(a+b)
→ Kc only changes when temperature changes
note: large Kc means we have more products than reactants
Distinguish between homogenous and heterogenous equilibria.
→ Homogenous equilibrium involves substances that are all in the same phase, including aq solutions where water is the solvent.
→ Heterogenous equilibrium involves substances in different phases
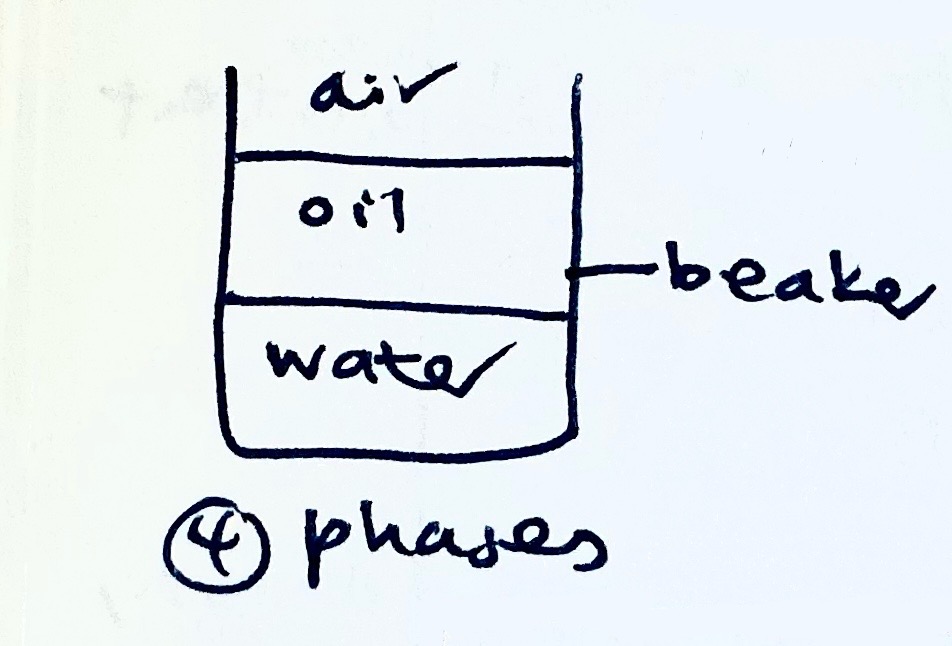
For heterogenous equilibria, what does the Kc expression not include?
→ conc of solids and liquids
note: if water is a solvent, omit it from the Kc expression. if water is a reactant, keep it in the Kc expression.

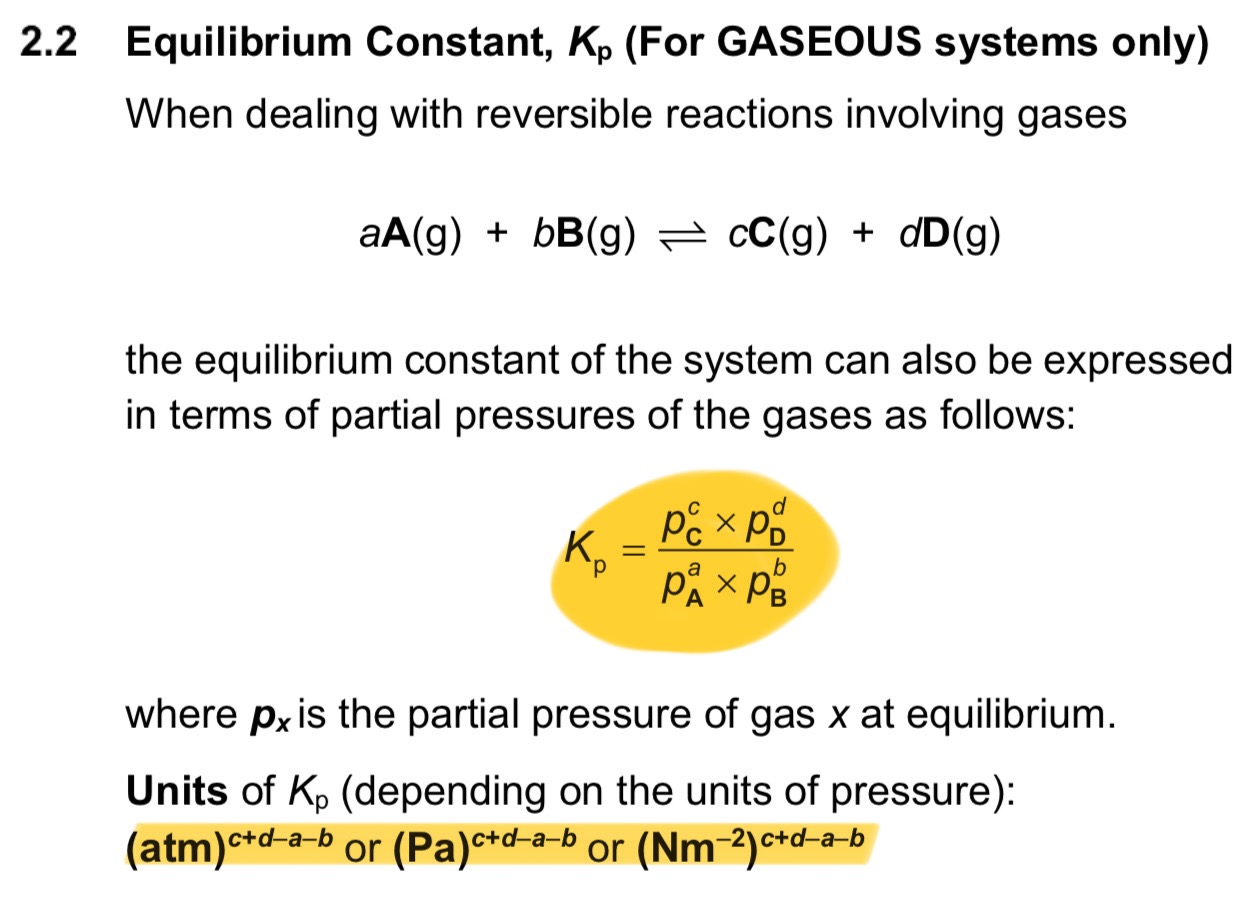
How do you express the equilibrium constant in gaseous systems? What are the units?
note: Kp expression only includes gases, not solids or liquids
Does Kc and Kp change with concentration, partial pressure, catalysts or temperature?
They change with TEMPERATURE only.
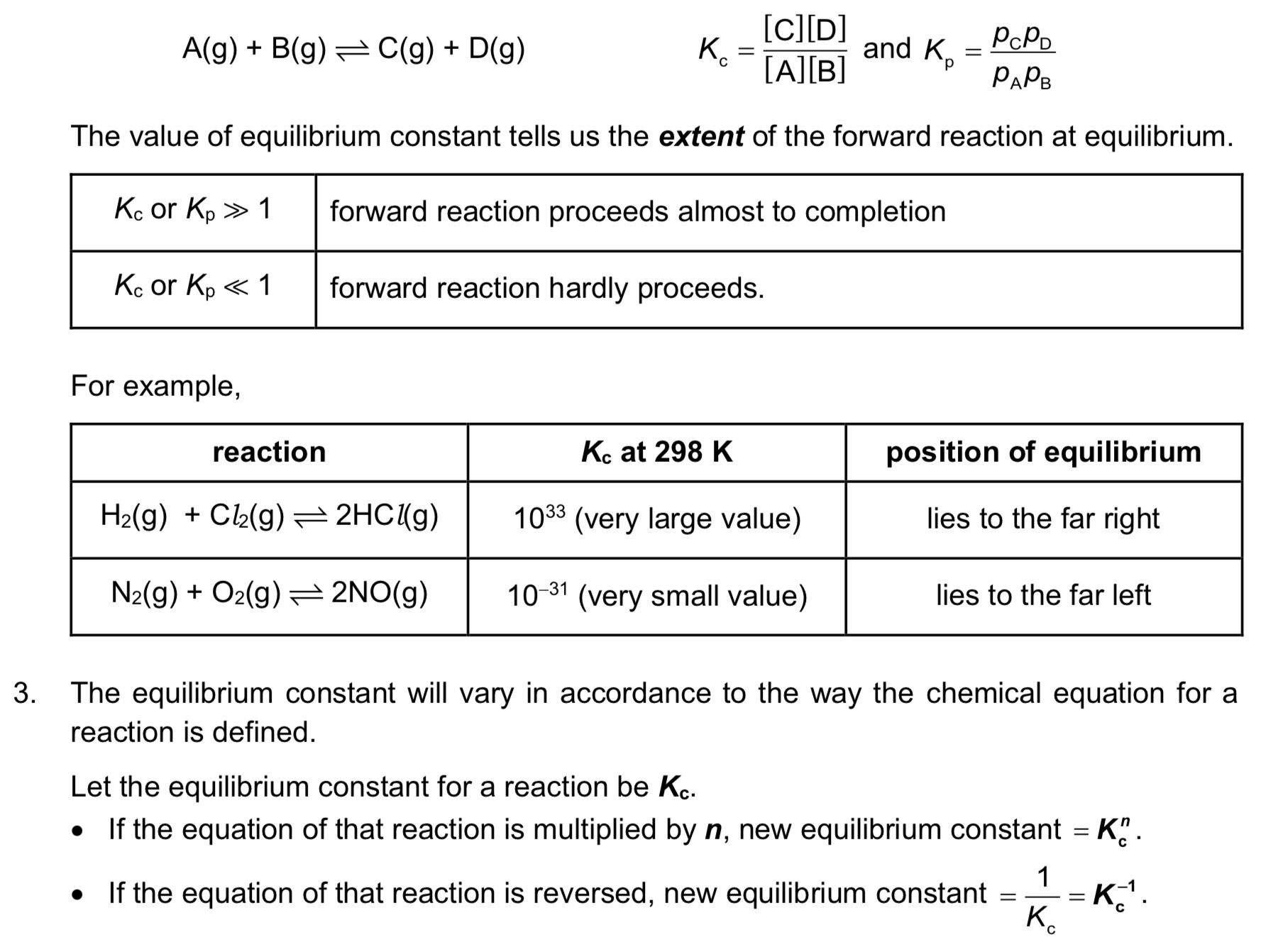
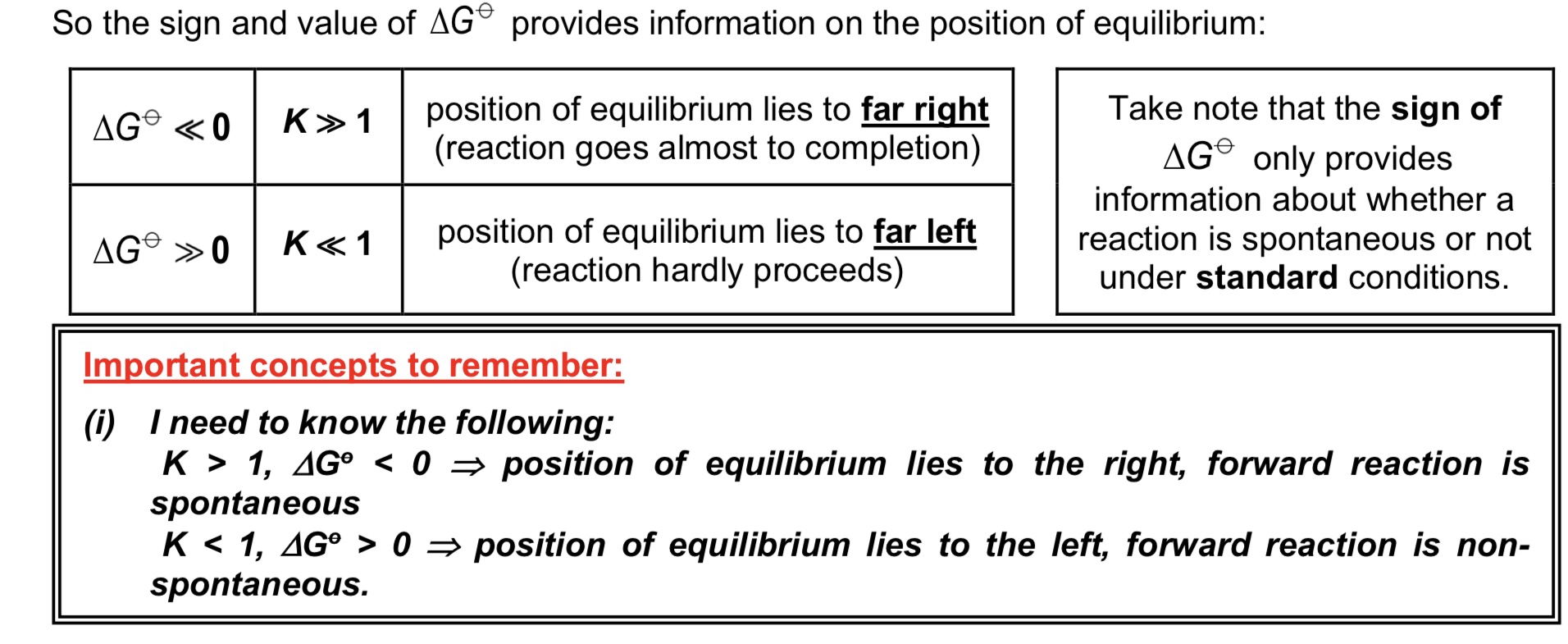
What does the position of the equilibrium tell us about the reaction?
→ POE lying to the right / Kc or Kp » 1 tells us that forward reaction proceeds almost to completion
→ POE lying to the left / Kc or Kp « 1 tells us that forward reaction hardly proceeds
Do equilibrium constants give any information on
rate of forward and backward reactions
time required for the reaction to reach equilibrium?
No.
note: equi constants can only be determined experimentally
What does it mean when △G° < 0 or △G° > 0 in a reaction?
Define Le Chatelier’s Principle.
LCP states that if a system in equilibrium is subjected to a change which disturbs the equilibrium, the system responds in such a way to counteract the effect of the change imposed, in order to re-establish the equilibrium of the system.
What is chemical equilibria affected by? What may changing these factors result in?
1) Concentration
2) Temperature
3) Catalyst
4) Pressure (gases only)
changing the above factors may result in
→ shifting of position of equilibrium (POE) to the left or right
→ change in Kc or Kp (ONLY FOR TEMP)
→ change in the rate at which equilibrium is established
Consider A (aq) ⇌ C (aq) in equilibrium. Describe what happens when [A] (aq) is increased.
→ When [A] (aq) is increased, the system will respond to remove some of A added.
→ POE shifts to the right and the forward reaction is favoured to form more C.
→ A new equilibrium is established.
![<p>→ When [A] (aq) is increased, the system will respond to remove some of A added.</p><p>→ POE shifts to the right and the forward reaction is favoured to form more C.</p><p>→ A new equilibrium is established.</p>](https://knowt-user-attachments.s3.amazonaws.com/0a8bbf60-14ec-4558-8e31-4f83802681db.jpg)
Pressure is inversely proportional to volume. Pressure ∝ 1/volume
When total pressure of a gaseous mixture is increased or decreased, how does the system respond?
→ When pressure is increased, the position of equilibrium shifts to the right to decrease the pressure by decreasing the number of gas molecules.
→ When pressure is decreased, the position of equilibrium shifts to the left to increase the pressure by increasing the number of gas molecules.
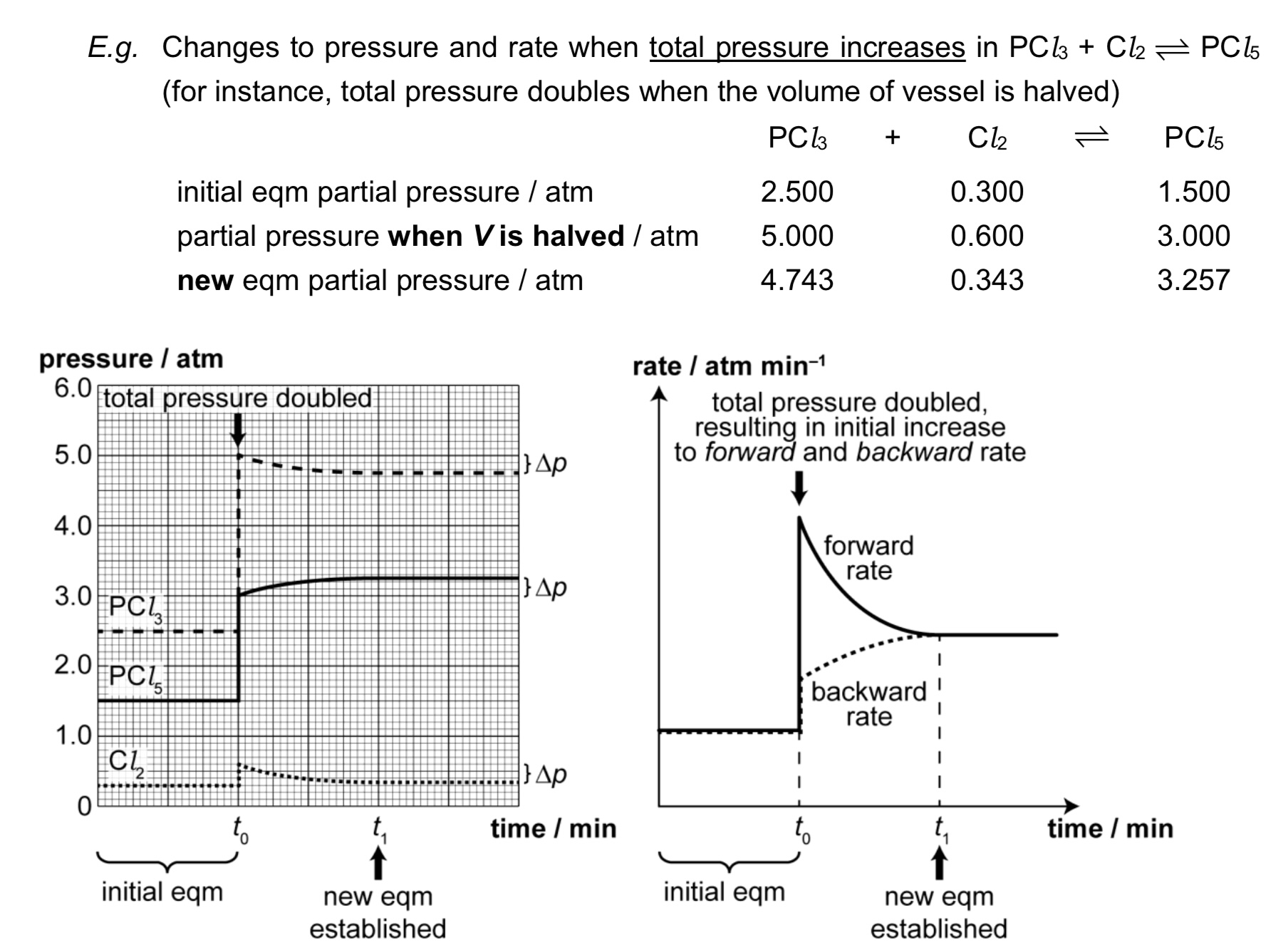
What happens when inert gas is added to the equilibrium system at constant pressure or constant volume?
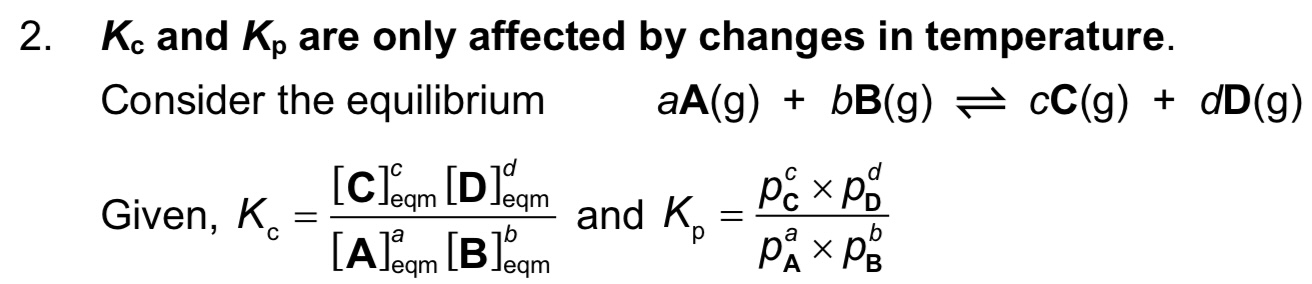
If △H is negative, how does temperature increase or decrease affect the equilibrium mixture?
→ As temperature increases, the POE shifts to the left to favour the backward endothermic reaction to absorb some heat, resulting in less products at equilibrium hence Kc / Kp decreases.
→ As temperature decreases, the POE shifts to the right to favour the forward exothermic reaction to produce some heat, resulting in more products at equilibrium hence Kc / Kp increases.
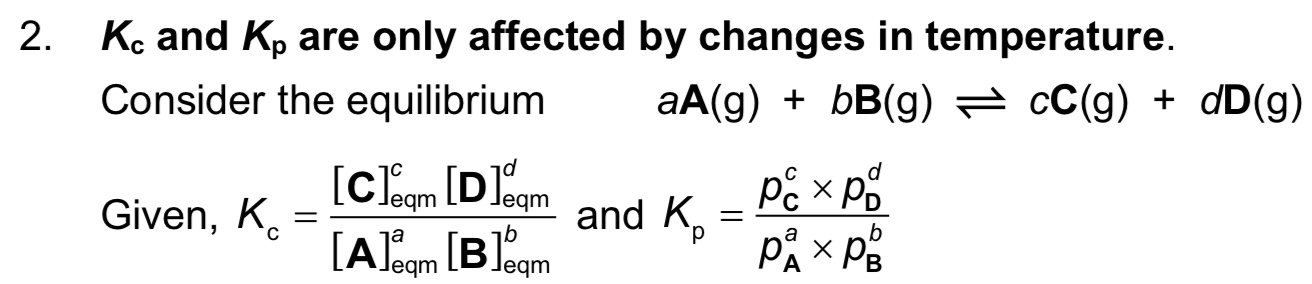
If △H is positive, how does temperature increase or decrease affect the equilibrium mixture?
→ As temperature increases, the POE shifts to the right to favour the forward endothermic reaction to absorb some heat, resulting in more products at equilibrium, hence Kc / Kp increases.
→ As temperature decreases, the POE shifts to the left to favour the backward exothermic reaction to produce some heat, resulting in less products at equilibrium, hence Kc / Kp decreases.
How does changes in temperature affect reactions with △H = 0?
For reactions with △H = 0, changes in temperature have no effect on the value of Kc or Kp since the POE is unchanged.
How do changes in temperature affect the forward and backward reactions?
→ Increase in temperature increases BOTH forward and backward reactions
→ Decrease in temperature decreases BOTH forward and backward reactions
Define “catalyst”.
A catalyst is a substance which increases the rate of reaction by providing an alternative reaction pathway with lower activation energy, without itself undergoing any permanent chemical change.
How does the addition of a catalyst affect an equilibrium?
→ A catalyst lowers the activation energy (Ea) of the forward and backward reactions by the same extent. The rate constant of the forward and backward reactions will be increased by the same extent (hence catalyst does not impact Kc or Kp).
→ Catalyst increases the rate of both forward and backward reactions by the same extent.
→ Presence of catalyst does not affect POE as system merely reaches equilibrium faster.
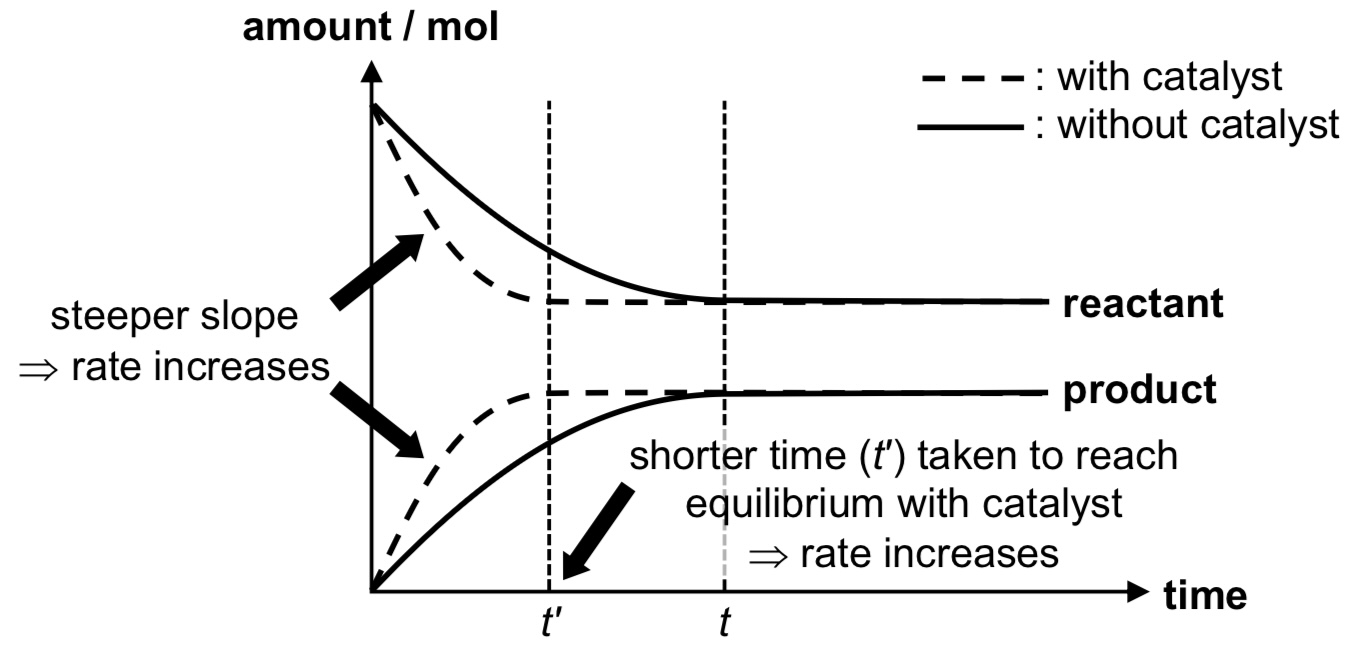
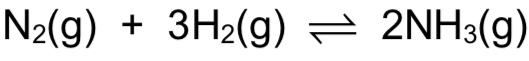
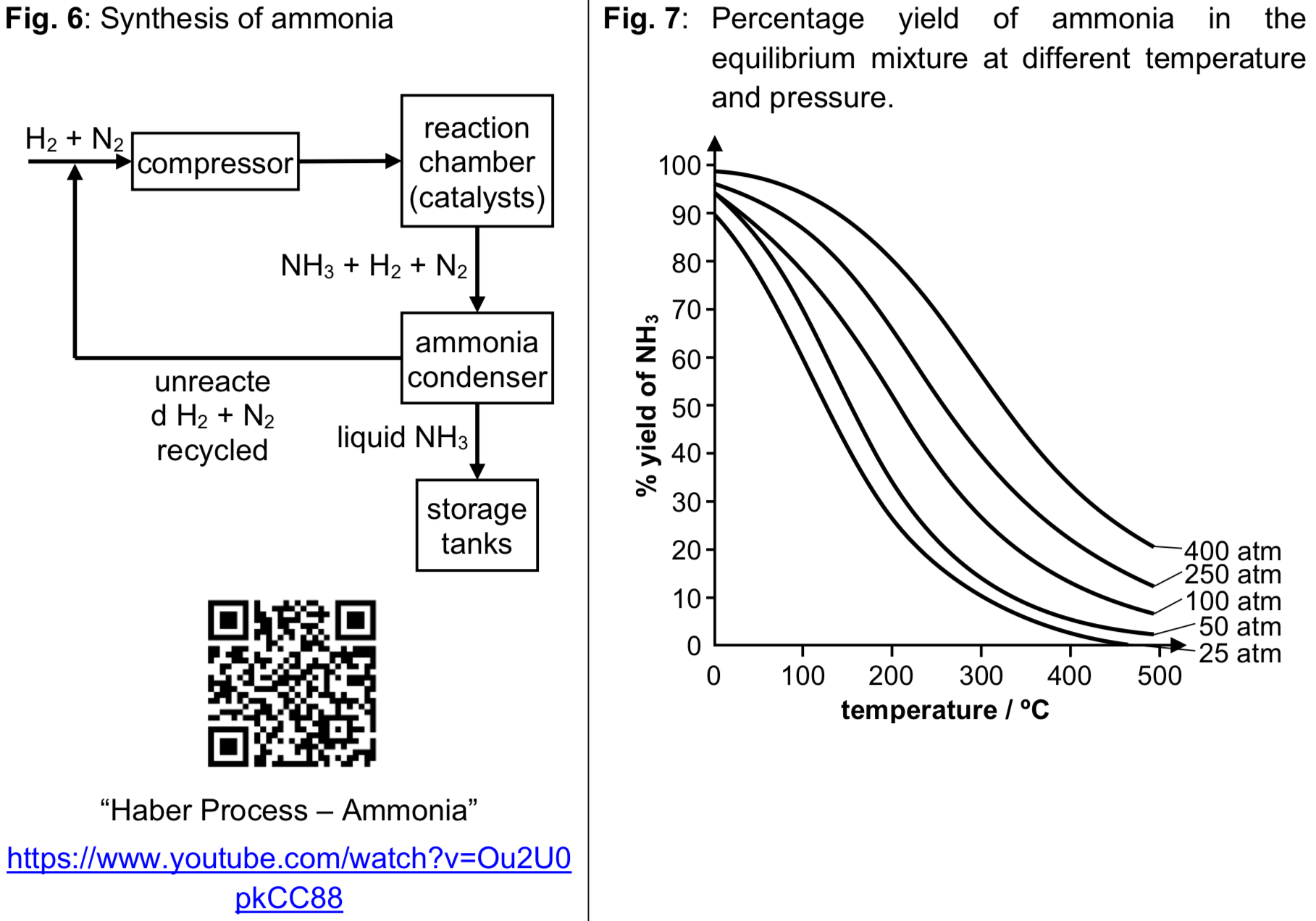
What are the conditions for the Haber process?
Temperature: 450°C → When temp decreases, POE shifts to the right to favour exo reaction to produce some heat, thereby increasing yield of ammonia. However reaction is slow at low temp due to low frequency of effective collisions between molecules, because of high energy needed to break strong N≡N bond. Hence compromise temp of 450°C is used to achieve reasonably high yield of ammonia at reasonably high rate.
Pressure: 250atm → When pressure increases, POE shifts to the right to decrease number of gas molecules to decrease the pressure, thereby increasing yield of ammonia. Reaction also reaches equilibrium faster at higher pressure. However high pressure will increase operating costs. Hence compromise pressure of 250atm is used to achieve reasonably high yield of ammonia at reasonably low costs.
Catalyst: Finely divided iron (increases surface area to volume ratio, SAVR) → Catalyst ensures equilibrium is reached faster, but has no effect on POE and hence no effect on ammonia yield.
→ must know Haber process is an exothermic reaction