Mammalian Reproductive Biology - Midterm Exam
1/170
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
171 Terms
Epigenetic change
Chemical modification on DNA or chromatin structure that alters the function of DNA without changing the actual DNA sequence. Germ cells (eggs and sperm) can carry these modifications and pass them onto offspring, which can affect generations of offspring.
What are the three major epigenetic markers?
DNA Methylation
Histone modification
Micro RNAs
What are introns?
Non-coding regions of the RNA strand; are not translated into proteins and need to be cut out.
Why are histones attracted to the DNA strand?
The phosphodiester bonds of the DNA are negatively-charged, which attracts the positive charges on histone proteins.
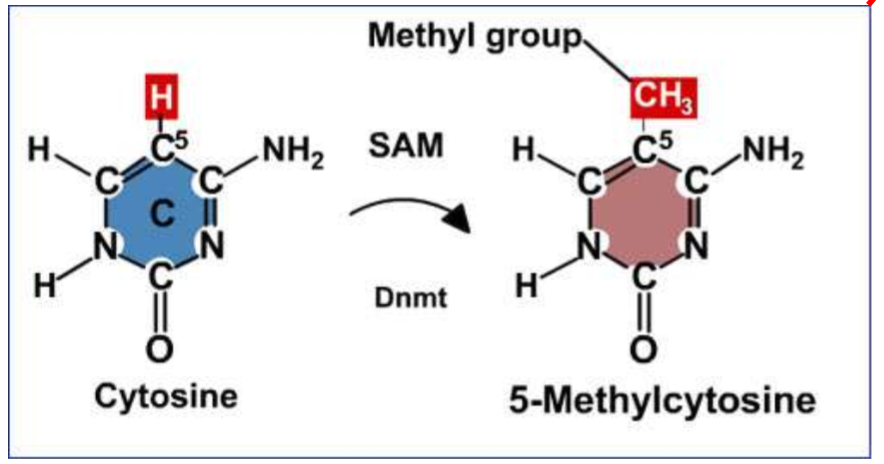
Chemical mechanism of DNA methylation
catalytic reaction to create 5’-Methylcytosine
DNMT adds a methyl group to cytosine using the methylating agent SAM → methyl group binds to the C5 region of the cytosine group
DNMT
DNA methyltransferase; responsible for adding a methyl group to DNA
CpG strand
A repetitive sequence of C and G bases; the region where DNA methylation takes place
SAM
S-adenosylmethionine → supplies a methyl group to cytosine during DNA methylation, and is turned into SAH (S-adenosylhomocysteine) after the reaction
HAT
Histone acetyltransferases → enzymes that acetylate conserved lysine amino acids
allow the DNA to be in a relaxed state → transcription factors able to bind
Methylation of globin genes
at 6 weeks of embryonic growth, the Epsilon(E)-globin promoter is unmethylated and active
at 12 weeks of growth, the E-globin promoter is methylated (inactive), and the gamma(y)-globin promoter is unmethylated (active)
this is because at 6 weeks, the bone marrow is not fully formed yet, so the liver makes hemoglobin; after 12 weeks, the bone marrow is able to create hemoglobin, so the E-globin promoter for the liver is switched off
HDAC
Histone Deacetylase → enzyme that removes acetyl groups from proteins, which can lead to gene repression
causes the DNA to tighten, so transcription factors are unable to bind
What are nucleosomes?
Units comprised of DNA wrapped around histone proteins
Linker DNA
The region of DNA between two connected histones
Histone 1 (H1) is the Linker → attracts other H1s to attract histones to each other, tightening the DNA strand
Methylation on Histone H3
depending on the position of the lysine on the tail, this activates or deactivates the histone
specific protein binding to H3 histone tails determines whether it will be methylated or unmethylated
MeCP2
Methyl-CpG Binding Protein 2
Binds to methylated cytosine residues in DNA, helping to silence gene expression
MECP2 gene is located on the X chromosome
HMT
histone methyltransferase → binds to histone tail and adds a methyl group; works jointly with MeCP2
Activation vs. Repression of Histones
Activation = H3K4me3, H3K36me3, H3 acetylation
Repression = H3K9me3, H3K27me3
What are the two checkpoints for developmental epigenetic programming?
The first checkpoint occurs around 1-2 weeks post conception (from the formation of the blastocyst to implantation)
The second checkpoint occurs during sex determination
These checkpoints represent windows when DNA methylation patterns are erased and re-established, making them particularly vulnerable to environmental influences
RNAPII
RNA Polymerase II; a multiprotein complex that transcribes DNA into precursors of mRNA and most small nuclear RNA (snRNA) and miRNA
RNAPII expressed = open chromatin → transcription ON
PRC2
Polycomb Repressive Complex 2
catalyzes the trimethylation of histone H3 at lysine 27 (H3K27me3), leading to the compaction of chromatin and the repression of gene transcription
suppression of this protein complex is linked to developmental disorders and various cancers
Where do cells with the highest morphogen concentration go?
Towards the center
Are epigenetic changes inherited through germ cells or somatic cells?
Germ cells, since they are able to be passed down to offspring. They cannot be passed on through somatic cells (all other body cells), as those are limited to the individual organism.
What are the three types of cell signaling?
Autocrine = a cell sends signals to target itself
Juxtacrine = short-range signaling (direct contact between cells)
Paracrine = long-range signaling (signals travel through extracellular space)
What do the position of cell aggregates depend on?
The surface tension (force/length) of each cell type
What is surface tension of cells determined by?
The presence of cell-adhesion molecules, such as proteins like cadherins
What happens when cells have the same surface tension?
When surface tension is the same, cells form boundaries by replacing cadherin with myosin.
Promoter Region
When methylated, transcription factors cannot bind → gene is silenced
Enhancer region
Located upstream of promoter; increases transcription likelihood when active
when methylated → decreased gene transcription
enhancer affinity influences promoter activity
What is the structure of chromatin?
147 base pairs of DNA wrapped around histones to form nucleosomes
Composition of a histone octamer
The protein core, composed of two each of H2A, H2B, H3, and H3. DNA wraps around this core, with the linker histone H1 binding to the linker DNA outside the core to further stabilize the structure.
Euchromatin
Relaxed/open state of chromatin → genes are accessible for transcription
Heterochromatin
Condensed/closed state of chromatin → genes are inaccessible and silenced
Function of microRNAs
Small RNAs that silence protein synthesis by binding to mRNA and segmenting it to degrade the strand of mRNA, which prevents translation
Regulation of miRNA
Produced in response to excessive mRNA levels → miRNA will break down the excess mRNA to prevent the overproduction of proteins
What is the clinical significance of miRNAs?
They can serve as biomarkers for disease states
Epigenetic checkpoint 1
Occurs 1-2 weeks post-conception
Occurs after blastocyst formation
Epigenetic changes will affect the somatic (body) cells of the developing embryo
Epigenetic checkpoint 2
Occurs during sex differentiation
Epigenetic changes will affect the germ line (reproductive cells)
Therefore, these alterations are transgenerational, meaning they can be passed on to the offspring
What is the high susceptibility period?
Between 3-8 weeks gestation
different organs have critical developmental windows
How is immune cell differentiation determined?
It is guided by morphogen gradients
What can chemical exposures disrupt?
Morphogen gradients
Cell differentiation phases
Organ development
Long term effects of epigenetic changes
DNA methylation changes during development can persist throughout life
dysregulated genetic marks can be activated OR regulated marks can be repressed
These changes can affect future generations through germ line modifications
Surface tension and cell sorting
Cells with lower surface tension surround cells with higher surface tension
this differential adhesion drives cell sorting and tissue organization
What are cadherins?
Cell adhesion molecules
form cadherin-cadherin bonds between adjacent cells
critical for maintaining tissue structure
What do integrins do?
They link cells to the extracellular matrix (ECM) and are essential for cell survival.
What would happen if integrins fail to bind to the ECM?
Cell-cell communication will be disrupted
Cells will not be able to survive, and will undergo apoptosis
Loss of actin cytoskeleton support
Cells may undergo epithelial-mesenchymal transition (EMT)
Function of actin filaments
Required for maintaining structural integrity of the cell → function as “anchors”
Inside the cell, ____ interact with cadherins and bind to the actin cytoskeleton (microfilaments). This bonding keeps the cells together.
Catenins
Epithelial-Mesenchymal Transition (EMT)
The process in which epithelial cells lose their polarity and adhesion, gaining migratory and invasive properties.
What kind of signaling is involved with EMT?
Paracrine signaling
Why is the epithelial-mesenchymal transition related to cancer?
Cancer cells use EMT to migrate and find oxygen-rich areas for survival.
offering more oxygen can be used as a way to keep the cancer cells in place long enough for them to be treated
Functions of morphogen gradients
Create concentration gradients across developing tissues
Tell cells their position in the developing embryo
Position determines cell fate and differentiation
Structure of Morphogen Gradients
High concentration = cells migrate toward the center/source
Low concentration = cells are at the periphery/edges
How can the disruption of morphogen gradients lead to birth defects?
Dysregulated gradients prevent proper differentiation
Changing gradients can cause abnormal cell positioning
Loss of proper cell-cell communication
What is a potential consequence of inner cell mass changes of the morphogen gradient?
Cells may repel each other, causing two separate cells masses on either side of the blastocyst → results in twin formation
What is a morphogen?
A diffusible biochemical molecule (paracrine) that can determine the fate of a cell by its concentration
Signal transduction cascades
Every cell has at least one type of receptor for morphogens
Cascades have master regulators that control downstream responses
If a master molecule is inhibited, the ENTIRE cascade will be disrupted
RTK Signaling Pathway
RTK = Receptor Tyrosine Kinase
Morphogens regulate differentiation through activation of RTK
Sequential phosphorylation events amplify the signal
What are four major signaling pathways?
JAK-STAT Pathway
WNT Pathway
SMAD Pathway
Notch Pathway
JAK-STAT Pathway
Function = Immune response regulation
Ligand binding activates JAK kinases → STAT transcription factors
What is the function of the WNT pathway?
Female sex development and organ development
WNT4 knockout → leads to impaired kidney development
What is the mechanism for the WNT pathway?
WNT ligand inhibits GSK3
Beta-catenin accumulates and enters the cell nucleus
Activates target gene expression
SMAD Pathway
Function: Cell growth, division, and development
Activated by TGF-beta superfamily ligands
Notch pathway
Function: Cell differentiation and fate determination
Requires direct cell-cell contact
What would happen if a ligand or receptor is disrupted in the WNT signaling pathway?
The embryo will fail to develop properly
Gene expression will not occur
Subsequent developmental responses will be blocked
Where does fertilization occur?
Ampulla of fallopian tube
What process must occur before fertilization?
Capacitation of sperm → removal of glycoprotein and seminal plasma coat
sperm undergoes biochemical changes in the female reproductive tract
Acrosome reaction
First step of fertilization:
Once bound to the zona pellucida, enzymes are released to help the sperm penetrate the oocyte.
IZUMO protein is expressed on the sperm membrane
What are the basic steps of fertilization?
Acrosome Reaction
Penetration of Corona Radiata
Binding to Zona Pellucida
Prevention of Polyspermy
Izumo-Juno Interaction
Completion of Meiosis
Penetration of Corona Radiata
Second step of fertilization:
Enzymes on the sperm head digest through the outer cell layer
Use hyaluronidase acid enzymes
Binding to the Zona Pellucida
Third step of fertilization:
Sperm binds to the glycoprotein layer surrounding an egg
ZP3 protein
What happens if the zona pellucida does not have enough oxygen (hypoxia)?
It will become too hard, preventing sperm from binding or breaking through → may result in delayed implantation
Can be reversed if oxygen is reintroduced
Prevention of polyspermy
Fourth step of fertilization:
Critical to prevent multiple sperm from fertilizing one egg
mechanisms: fast block (electrical) and slow block (cortical granule release)
What happens if polyspermy DOES occur?
The egg will become “invisible” due to too many chromosomes
IZUMO-JUNO Interaction
Fifth step of fertilization:
IZUMO ligand on sperm binds to the JUNO receptor on egg
This binding is required for membrane fusion → the Izumo protein mediates the adhesion and subsequent fusion of sperm with the egg cell membrane
Completion of Meiosis
Sixth step of fertilization:
Egg completes meiosis II after fertilization
Sperm prepares by removing flagella and comes closer to female pronucleus
Then, both undergo mitotic division
Why does the corpus luteum stop producing progesterone after a while?
The embryo is able to start producing its own progesterone.
Hyaluronidase acid enzyme
Function: Digest the glycoprotein layer; helps with penetration into the corona radiata
Embryonic development timeline
0-3 weeks = early development (cleavage, gastrulation)
4-8 weeks = period of embryonic organogenesis
9-38 weeks = fetal period
What event marks “Day 1” of development?
Fertilization → wherein the sperm meets the egg in the fallopian tube
Events during Day 2 of development:
Cleavage continues → Two-cell stage
Rapid mitotic divisions
maternal mRNA degrades as the zygotic genome activates (able to produce its own mRNA)
Events during Day 3 of development
Morula stage (16-32 cells; form a solid ball)
Events during Days 4-5 of development:
Early blastocyst forms on Day 4, while late blastocyst forms on day 5.
fluid-filled cavity (blastocoel) develops
two distinct cell populations form
Events during Day 7-7.5 of development:
Two layers of the trophoblast form: Cytotrophoblast and Syncytiotrophoblast
Two layers of inner cell mass form: Hypoblast and Epiblast
What two layers does the trophoblast split into?
Cytotrophoblast → Inner layer
Syncytiotrophoblast → Outer layer (invades the uterine lining)
What two layers does the embryoblast split into?
Epiblast (primitive endoderm) → eventually forms the three germ layers (endoderm, ectoderm, mesoderm)
Hypoblast → eventually forms the yolk sac and extraembryonic tissues
What two layers does the extra-embryonic mesoderm split into?
Somatic layer (Parietal layer) → Forms the body wall structures
Splanchnic layer (Visceral layer) → Forms the gut-associated structures
What are the two cavities that form during embryonic development?
Yolk sac
Amniotic cavities
Events during Day 13 of development:
Lacunar channels form in the syncytiotrophoblast → allow maternal blood to flow around the embryo
Cytotrophoblast maintains structural integrity
What does the mesoderm differentiate into?
Bones
Connective tissue
Urogenital system
Cardiovascular system
Maternal-fetal gas exchange
Occurs across the placental interface
Oxygen and nutrients from the mother are passed to the fetus
Carbon dioxide and waste from the fetus are passed to the mother
Primordial Germ Cells (PGCs)
The precursors to all reproductive cells, first formed in the yolk sac during early embryonic development
What do the PGCs differentiate into?
Males → spermatogonia (sperm stem cells)
Females → oogonia (egg precursors)
What is the basic function of somatic cells in gonadal development?
They provide structural and nutritional support to germ cells
What does the ectoderm differentiate into?
Skin
Central and peripheral nervous systems
Eyes
Internal ear
Neural crest cells → bones and connective tissue of the face + part of the skull
What does the endoderm differentiate into?
The gut and gut derivatives (liver, pancreas, etc.)
What are the three domains of the ectoderm?
Surface ectoderm → primarily epidermis
Neural crest → peripheral neurons, pigment, facial cartilage
Neural tube → brain and spinal cord
What are the gonadal somatic cells in males?
Sertoli cells → support sperm development; secrete Anti-Mullerian Hormone (AMH)
Leydig cells → produce testosterone
What are the gonadal somatic cells in females?
Granulosa cells → support egg development
Theca cells → produce androgen precursors for estrogen synthesis
The Indifferent Stage
Early embryos have TWO duct systems that can develop into either male or female reproductive tracts
Wolffian ducts (mesonephric ducts) → become male internal structures if preserved
Mullerian ducts (paramesonephric ducts) → become female internal structures if preserved
Where did the Wolffian and Mullerian tubes develop from?
Both develop from the intermediate mesoderm along the genital ridge