Intermolecular forces, liquids
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Difference in state of matter is due too..?
distance between particles
solids locked in place
slight disorder, particles move (slower)
gases - total disorder , much empty space
state of substance depends on…?
kinetic energy of particles ( temp dependent) - higher KE → faster → particles overcome attractive forces ( keeps particles separate)
The strength of attraction between particles
two sides of tug a war kinda
intermolecular force s
attraction between 2 separate molecules
controls physical properties
intramolecular forces
bonds between atoms in a molecule ( intra=within molecule)
intramolecular is stronger than intermolecular forces
another name for intermolecular forces
van der waal forces
ions vs dipoles
ion = charge
dipole = partial charge ( overall atom has neutral charge)
ion-dipole forces
attraction between an ion (Na+) and a polar molecule ( a molecule with a charge of 0, but uneven electron distrubtion (probs due to electronegativity) leading to dipole moment
ions full charge is attractred to part of the partial charge on the dipole
one of the strongest IMF forces cause we’re using a full charge
need an ionic compound
dipole - dipole forces
attraction of polar molecuels with permanent dipole moments, the partial negative ends of polar molecules are attracted to the partial positive ends of other polar molecules ( not as powerful as ion dipole)
higher dipole moment leads to higher boiling point and higher melting point ( harder to seperate the molecules )
melting point/boiling point
amount of energy u have to put in to separate the molecules
hydrogen bonding
especially strong form of dipole-dipole interaction
occurs when hydrogen is bonded to N, O or F - due to their high electronegativity
when they bond, the electronegative atoms, create an insanely polar bond with hydrogens 1 electron; esenittaly leaving hydrogens nucellus exposed
why does water form such a strong hydrogen bond
cause each water molecule can donate 2 Hs and accept 2 Hs, making them fit together like puzzle pieces; forming a strong network
4 hydrogen bonds per oxygen leads to a full tetrahedral molecule, when these freeze they form a chain of tetrahedral molecules, creating an open and gap filled structure; which is why ice is less dense than water
London Dispersion Forces
for an instant in time, electrons can be asymmetrical arranged around the nucleaus such that the atom is polarized → instantaneous dipole → which induces a dipole in a neighbouring atom
LDF are present in all molecules regardless of polarity
affected by shape: longer molecules have stronger LDF, than spherical molecules cause of their increased surface area
also affected by molecular weight; more electrons means easier for an instatnatous dipole to occur
scale with weight, alter with form
Viscosity
resistance to flow units: Pas ( equivalent to kgm-1s-1)
higher viscosity = stronger intermolecular forces ( harder to seperate)
higher viscosity = molecular shape is easier to entangle
what creates surface tension
at the surface of a liquid there is an imbalance of intermolecular forces ( no forces above surface) -
this creates a tight surface as forces are pulling down and away from top( surface tension)
it is caused by cohesive forces ( binds molecules to like molecules)
surface tension units
units Jm-2 - surface tension is the energy cost of creating more surface area ( work required to create more surface area)
how resistant a liquid is to increasing its surface area ( takes energy to pull molecules apart to make surface bigger)
examples of strong surface tension
water droplets or bugs walking on water ( strong hydrogen bonds)
interfacial behaviour - cohesive forces
describes how 2 phases interact
cohesive forces : binds like molecules to one another
visually liquid beads up
( IMFs between like molecules have to be strong)
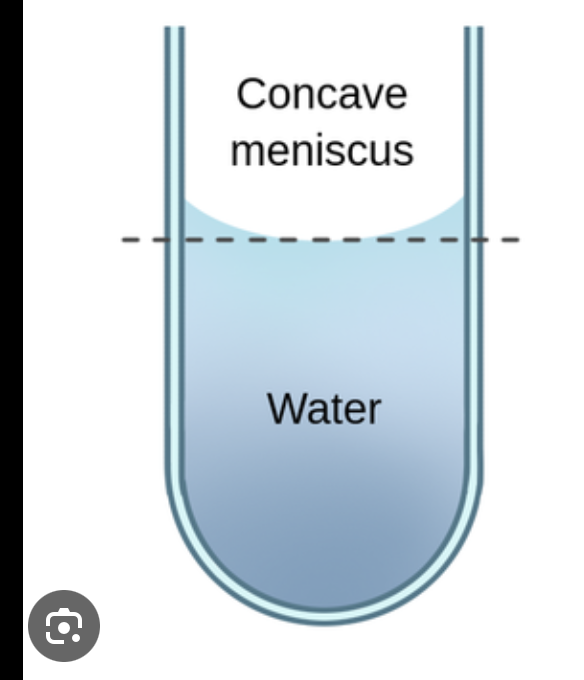
what creates a concave surface
when adhesive forces are greater than cohesive forces - molecule binds to surface as much as possible
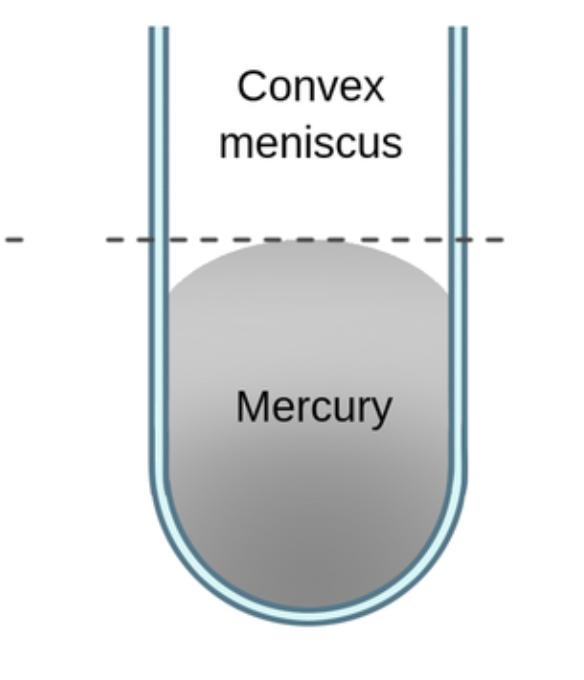
what creates a convex surface
cohesive forces are greater than adhesive forces, liquid does NOT bond to surface bonds internally instead
interfacial behaviour - adhesive forces
Adhesive Forces: binds molecules to the surface ( liquid can form strong IMFS with surface)
liquid spreads out
how to tell if cohesion of adhesion wins ( might not be needed for exam)
like sticks to like
polar liquid sticks to polar surfaces
non polar liquids stick to non polar surfaces
a mix of both? cohesion wins
capillary action
ability of a liquid to rise or fall in a tube without external forces
due to a mix of adhesive and cohesion forces
adhesive forces draw liquid along sides of tube ( surface)
cohesion forces between liquid molecule, pulls remaining molecules along with first molecules
adhesive forces are the catalyst but cohesion forces carry everything else along too
evaporation
occurs when molecules near surface of liquid get enough energy to overcome IMF and transition from liquid to gas
ease of evaporation dictates both boiling point and vapour pressure
evaporation in open system vs closed system
open system: molecules evaporate and are removed from system ( float away)
closed system: molecules evaporate but condense back at the same rate, system is in equilibrium ( this defines vapour pressure )
vapour pressure
pressure exerted by a liquids vapour when its in eq with the liquid in closed container
weak IMFs→ at that temp more molecules escaping into gas → more molecules exert more pressure → higher vapour pressure
strong IMFs → at that temp less molecules escape into gas → exert less pressure in gas phase ( cause theres less of them) → low vapour pressure
Vapour pressure measures how many molecules are in the gas phase at that temp (at equilibrium). ( high vapour pressure means more → weaker IMFs)
when is boiling point reached
when vapour pressure = external pressure
when these two are equal , bubbles of vapor can form inside liquid not just on surface, → liquid boils
normal boiling point?
when liquids vapour pressure is 1 (atm) atmosphere = 760mm Hg
normal = standard atmospheric pressure
what happens to boiling point at higher altitudes?
air pressure decreases as altitude increases,
at higher alitidue liquids boil at lower temp
however this means it takes longer for food to cook, because the liquid is cooler so it has less energy and cooking/heating things up takes longer
volatile liquids
evaporate easily
have higher vapour pressure
Phase Change Diagram
plots state of matter as function of pressure and temp
sublimation: solid turns directly into gas (sub means up or sky)
deposition: gas changes directly into a solid (depositing means leaving behind)
Freezing: Liquid turns to solid
Melting: solid turns to liquid
Boiling: liquid turns to gas
condensing : gas turns to liquid
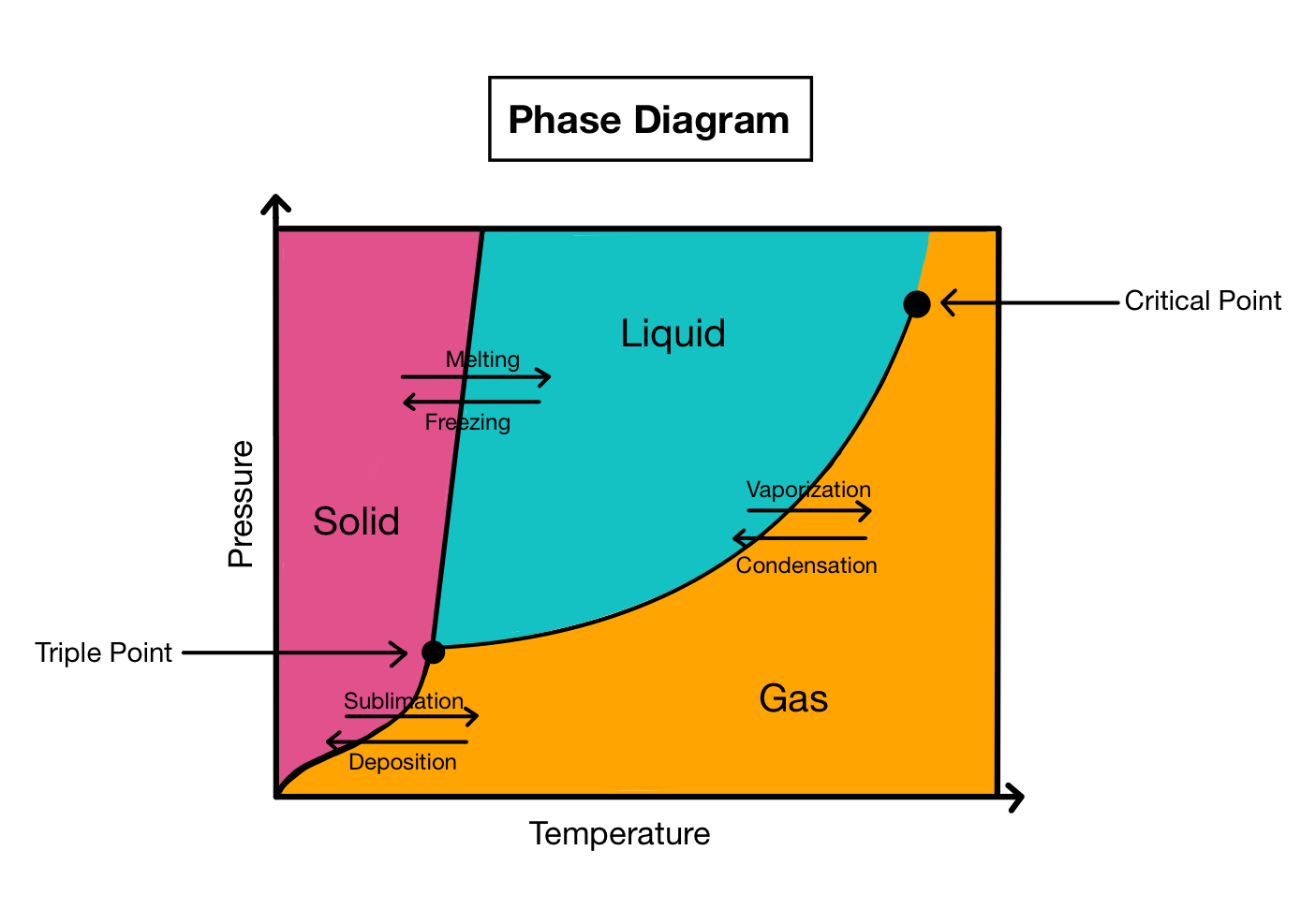
triple point
all three phases present
super critical fluid
has properties between liquid and gas
ex: SC CO2 Used to extract caffeine from coffee
what determines density
density = mass/volume , liquid water has more density than solid ( due to the shape of the solid having gaps)
liquid crystals
molecule is above melting point ( should be liquid) but exhibits solid characteristics
a true liquid is isotropic - molecules point in every possible direction ; not the case for liquid crystals
its an opaque liquid with crystalline ordering but also ability to flow
usually long and rod like - after second melting point are isotropic ( randomly oriented - become ‘true liquids’)
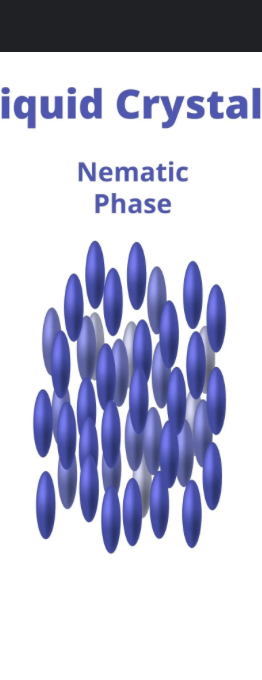
nematic liquid crystals
crystals ordered along the long axis of molecule ( one direction)
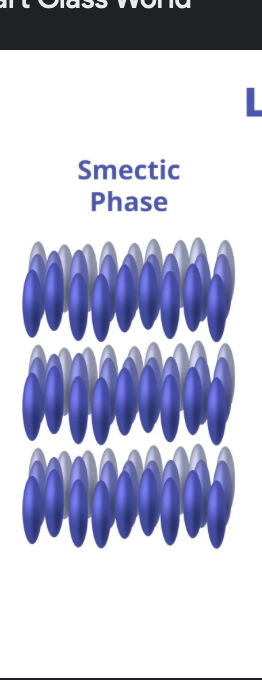
smectic liquid crystal
ordered along long axis AND another dimension ( sheets break it up?)
cholesteric liquid crystals
ordered along long axis and in twisted layers?

Semi conductors:
Si, GaAs, InP, CdSe
average of 4 VE
insulators
glass, air, rubber , wood, most plastics ( polymers)
addition polymers ( how to identify)
double bonds that open and link
all C in back bone
Condensation polymerization
eliminates small molecule ( product)
not just carbon in the back bone ( monomers contain multiple? functional groups)
nano materials
go from scale of 1-100 nm
at scale materials show different behaviour then at regular size \
semi conductors: at this range are called quantum dots , band gap increases as particle size decreases
metals: at this range have deep/stain glass colors
graphene:, single, unrolled sheet of graphite semi metal with record thermal capacity, very strong
graphite = many layers of graphene ( graphene is a single layer of carbon)
carbon nanotubes:
graphene sheet rolled up and capped at both ends
very strong
why is silicon used for wafers/chips
Si is abundant, cheap and can grow enormous perfect crystals
non toxic, can be chemically protected with SiO2 ( bonding with air protects outer layer)
How do solar energy cells work?
uses photo conductivity and semi conductors
joins n-type and p-type silicon ( different types of semi conductors)
shine light with appriopiate wave length ( photo conductivity) on a semi conductor , and electrons are promoted to conduction band ( making material more conductive)
How do light emitting devices (LEDs) work?
also p-type and n-type semi conductors joined
a voltage is applied and electrons in the conduction band from n-type side recombine with holes in the p-type side, releasing photons who have energy equal to the band gap
why are organic LEDs better?
lighter, more flexible, brighter, energy efficient, purely black no light reflected
enabled creation of curved displays
some problems with lifter of devices
poly sugar creates what
paper, cotton
chitin
a poly glucose with an amide group - chitin makes up exoskeletons
plastic types ( reshaped vs not)
thermoplastics can be reshaped
thermosetting plastic materials can not