IB Chem Unit 7 (Phase diagram and change)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Solid
Molecules packed tightly together
Defined volume, defined shape
The temperature and kinetic energy are low
Liquid
Molecules packed moderately-tightly together
Defined volume, undefined shape
Takes the shape of its container
Temperature is higher, kinetic energy is medium
Gas
Molecules are far apart
Undefined volume, undefined shape
Temperature and kinetic energy are high
how to determine the phase of a substance
The phase of a substance depends on temperature and pressure
Temperature is a measurement of the average kinetic energy in a sample
Therefore, the hotter a substance is the faster the molecules move
Adding or removing heat to/from a substance causes phase changes
From solid to liquid
Solid → Liquid
Melting
Endothermic
Liquid → Solid
Freezing
Exothermic
Liquid → Gas
Vaporization
Endothermic
Gas → Liquid
Condensation
Exothermic
Solid → Gas
Sublimation
Endothermic
Gas → Solid
Deposition
Exothermic
Phase Diagram
Phase diagrams represent phase changes as a function of temperature & pressure.
Relate temperature and pressure to physical state
Normal boiling/melting point pressure
101 kPa
1.0 atm
760 mmHg
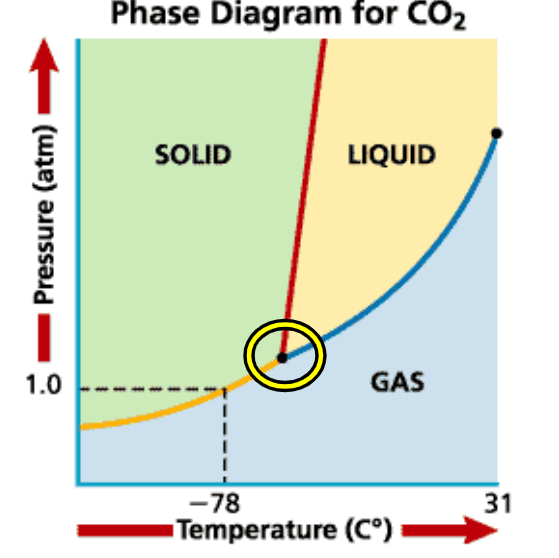
Triple Point
Point on the phase diagram where solids, liquids and gasses all exist at the same time.
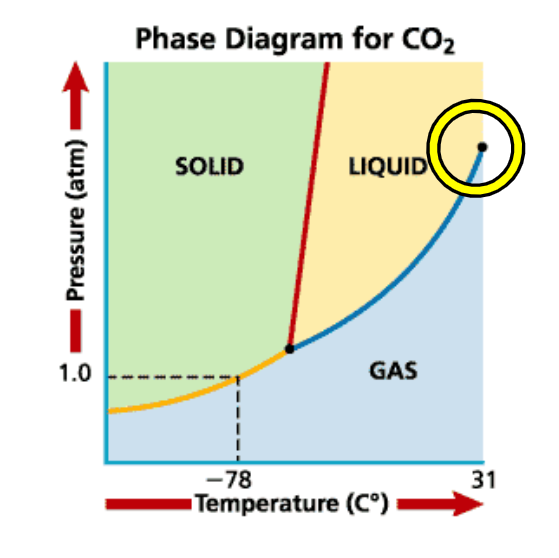
Critical Point
Point where the liquid can no longer be distinguished from a gas
Vapor cannot be liquefied
Occurs at extremely high temperature and pressure