VSEPR Theory and Electron Pair & Molecular Geometries
1/15
Earn XP
Description and Tags
basically js a rundown of the electron geometries of anything ap may ask according to vsepr theory
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Linear, one electron domain
Formed when one bond forms between two atoms. Has angle of 180° off central atom; forms a hybridization orbital of s.
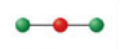
Linear, two electron domains
Formed when two bonds form off the central atom. Has angle of 180° off central atom; forms a hybridization orbital of sp.
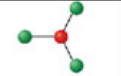
Trigonal Planar, three electron domains
Formed when three bonds form off the central atom with no lone pairs. Has angle of 120° off central atom; forms a hybridization orbital of sp².
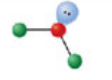
Bent, three electron domains
Has an electron pair geometry of trigonal planar. Formed when two bonds form off the central atom, and the atom has one lone pair. Has an angle of 109.5° off central atom; forms a hybridization orbital of sp².
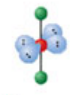
Tetrahedral, four electron domains
Formed when four bonds form off the central atom with no lone pairs. Has an angle of 109.5° off the central atom; forms a hybridization orbital of sp³.
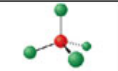
Trigonal pyramidal, four electron domains
Has an electron pair geometry of tetrahedral. Formed when three bonds form off the central atom, and the atom has one lone pair. Forms a hybridization orbital of sp³.
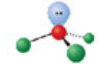
Bent, four electron domains
Has an electron pair geometry of tetrahedral. Formed when two bonds form off the central atom, and the atom has two lone pairs. Forms a hybridization orbital of sp³.
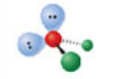
Trigonal bipyramidal, five electron domains
Formed when five bonds form off the central atom with no lone pairs. Can have angles of 90° or 120° (depends on which bond) off the central atom.
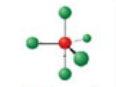
Sawhorse, five electron domains
Has an electron pair geometry of trigonal bipyramidal. Formed when four bonds form of the central atom, and the atom has one lone pair.
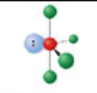
T-shaped, five electron domains
Has an electron pair geometry of trigonal bipyramidal. Formed when three bonds form off the central atom, and the atom has two lone pairs.
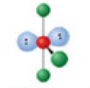
Linear, five electron domains
Has an electron pair geometry of trigonal bipyramidal. Formed when two bonds form off the central atom, and the atom has three lone pairs.
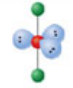
Octahedral, six electron domains
Formed when six bonds form off the central atom with no lone pairs. Has an angle of 90° off the central atom.
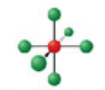
Square pyramidal, six electron domains
Has an electron pair geometry of octahedral. Formed when five bonds form off the central atom, and the atom has one lone pair.
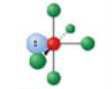
Square planar, six electron domains
Has an electron pair geometry of octahedral. Formed when four bonds form off the central atom, and the atom has two lone pairs.
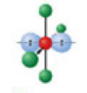
T-shaped, six electron domains
Has an electron pair geometry of octahedral. Formed when three bonds form off the central atom, and the atom has three lone pairs.
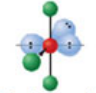
Linear, six electron domains
Has an electron pair geometry of octahedral. Formed when two bonds form off the central atom, and the atom had four lone pairs.