IE 4: Personalized Medicine in Oncology
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
What is a mutation?
A mutation is an alteration in a DNA nucleotide sequence of the genome
Can occur in single nucleotides or large segments of DNA
Linked to a variety of factors, such as hereditary, exposure to carcinogens, age, etc.
What is the differences between mutations vs polymorphism?
Mutation:
Very rare (less than 1% of population)
Direct cause of disease
Polymorphism:
Widely prevalent
Contribute to genetic diversity
Polymorphisms do NOT directly cause a disease but can do what? What is an example of this?
Can influence therapy
Ex:
Polymorphisms: TPMT/NUDT15 and DPYD
Do not contribute to disease pathology of cancer, however, can influence therapy tolerance and efficacy
Mutations: CFTR
Cystic fibrosis
What are some germline polymorphisms that impact cancer therapy?
TPMT/NUDT15 and 6MP
DPD and 5-FU
How does 6-MP and TPMT/NUDT15 affect therapy?
Acute lymphoblastic leukemia (ALL) treatment: 6-MP
Polymorphisms of TPMT or NUDT15 with reduced metabolic rate of 6-MP → lead to increased risk of side effects
Bone marrow suppression, leukopenia, thrombocytopenia
How does DPD and 5-FU affect therapy?
DPD is the enzyme involved in 5-FU and capecitabine metabolism and degradation
DPD deficiency is an autosomal recessive disorder
Reduced DPD activity leads to reduced metabolism of 5-FU and results in high concentrations of 5-FU in the body
Results in severe 5-FU toxicity
Where do germline mutations occur?
Occur in the germline, tissues that develop sex cells
Germline mutation can be transmitted to what? What happens to when it gets passed on?
May be transmitted to some or all progeny
Mutation will be passed on the next generation if the mutant sex cell participates in fertilization
Resulting mutation found in all cells of the offspring
What are germline mutations the cause of?
Cause of rare diseases
Cystic Fibrosis (CFTR gene)
Most common mutation is an amino acid deletion, F508
How are germline mutations distinguished from germline polymorphisms?
They are very rare
They are the direct cause of disease pathology
What are somatic cells?
Those that are not gametes
When do somatic mutations occur?
Random mutations that occur during mitotic cell divisions
Somatic mutations are only found where?
Only found in tissue where it occurred and resulting cells from mitosis
Somatic mutations are not transmitted where? They are the source of genetic mutations leading to what?
Not transmitted to progeny (not inherited)
Source of genetic mutations leading to cancerous growth and treatment resistance
What is cancer?
Cancer is a molecular disease that can develop anywhere in the body
Uncontrollable growth of cells that can invade surrounding tissues or spread to other organs (metastasis)
What are biomarkers? How can they be used?
Biological molecule found in blood, other body fluids or tissues that is a sign of a normal or abnormal process, or of a condition or disease
May be used to see how the body responds to a treatment for disease or conditions
May be used to provide information about prognosis
What do tumor suppressor genes regulate and prevent? What are many involved in? When mutated, what can happen?
Regulate cell growth and division
Prevent development of cancer
Many involved in DNA repair
When mutated, cells can grow uncontrollably and lead to cancer
What are examples of tumor suppressor genes?
BRCA
TP53
BRCA1 and BRCA2 do what? Mutation in either gene does what?
Produce proteins that help repair damaged DNA
Mutations in either gene increases the risk of developing breast and ovarian cancer
How many copies in BRCA1 and BRCA2? What is the process when it gets mutated?
Two copies of each gene – one inherited from each parent
BRCA1/2 mutation (+) → inactivation of tumor suppressor → uncontrolled cell growth
What can treat the BRCA1 and BRCA2 mutations?
PARP inhibitors: olaparib, rucaparib, niraparib, talazoparib
What does the TP53 code for? What is it used for?
Codes for tumor protein 53 inside of the cell nucleus
A tumor suppressor gene that helps control cell division and cell death
In DNA damage, what does p53 determine? What happens when TP53 has a mutation?
DNA damage → p53 determines either DNA repair or apoptosis
If DNA repaired → p53 activates other genes to help fix damage
If apoptosis → p53 prevents cell division and signals apoptosis
TP53 mutation (+) → lack of cell growth control and apoptosis control for damaged DNA → uncontrolled cell division
Inherited TP53 gene mutations are often found where?
Inherited TP53 gene mutations often found in Li-Fraumeni syndrome
Genetic condition that causes increased risk for developing rare tumors (soft tissue sarcomas, osteosarcomas)
Oncogenes are usually what? How are they activated?
Oncogenes are usually kinases, growth factors or transcription factors
Oncogenes are activated by point mutations, amplification, and gene fusions
In oncogenes, what are examples of point mutations and amplifications, gene amplification, and chromosomal translocation?
Point Mutations and amplification
Growth factor receptor network
EGFR
KRAS
BRAF
ERBB2 (HER2)
Gene Amplification
ERBB2
Chromosomal Translocation
Philadelphia chromosome (eg; BCR-ABL)
What does the presence of EGFR protein (EGFR+) only, indicate?
Presence of EGFR protein (EGFR+) only indicates worse disease prognosis
Determined by immunohistochemistry
Higher the protein, worse the prognosis
Overexpression of EGFR protein serves as what?
Overexpression of EGFR protein serves a biomarker for EGFR-targeting monoclonal antibody(Mab):
Cetuximab, panitumumab
EGFR+ alone does not predict what?
EGFR+ alone does not predict therapeutic response to EGFR inhibitors (EGFRI)
KRAS mutations leads to EGFRI resistance
Not effective if EGFR mutations are present
EGFRI tyrosine kinase inhibitors (TKI)
EGFR mutation occurs in how many with what? The mutation is to the portion of DNA in cells responsible for what?
Occurs in ~15% of people with lung cancer in the US
Mutation to the portion of DNA in cells that are responsible for developing EGFR proteins
Activate tyrosine kinase (TKI) domain
What are examples of EGFR mutations?
EGFR mutations:
L858R mutation (+) → predictive of treatment benefit from EGFR TKI therapy
Erlotinib, gefitinib, lapatinib, afatinib
T790M mutation (+) → may lead to resistance of EGFRI
Osimertinib-3rd generation EGFR TKI is indicated for EGFR T790M mutation
For the HER2 receptor or ERBB2 gene, amplification shows what? It is over expressed in what patients?
Poor prognostic factor
Overexpressed in 15-20% breast cancer patients
What is first line treatment of HER2?
Trastuzumab (Herceptin)
Where is the KRAS found?
Found downstream of the EGFR signaling network
Mutations in the KRAS gene is associated with what? Presence of KRAS mutation is mutually exclusive of presence of what?
Resistance to EGFRI-TKI therapy
Presence of KRAS mutation is mutually exclusive of presence of EGFR mutation
If a patient is BRAF V600E mutation positive, what can help benefit?
Significant long term treatment benefits from BRAF inhibitors
What are the BRAF inhibitors?
Vemurafenib, trametinib, dabrafenib, encorafenib
The presence of BRAF mutation is exclusive of what?
Presence of BRAF mutation is exclusive of KRAS mutation
Co-mutation for both BRAF and KRAS is extremely rare
The BCR-ABL is an abnormal gene found where? What does it code for/
Found in chronic myeloid leukemia (CML) cells
Codes for tyrosine kinase protein for cell growth and reproduction
If a patient is BCR-ABL positive, what happens?
CML cells grow and divide uncontrollably
What is treatment that targets BCR-ABL?
Imatinib
Nilotinib
Higher expression of ERCC1 (DNA repair gene) causes what? What are platinum drugs
Causes resistance to platinum molecules (and drugs)
Platinum drugs:
Oxaliplatin, cisplatin, carboplatin
Triggers cell death by crosslinking of DNA
What should be considered without platinum compounds?
Alt chemo regimens
What is an inhibitor of Thymidylate Synthase (TS) which causes what?
fluorodeoxyuridylate (FdUMP) is an inhibitor of TS → DNA damage →
cell deathFdUMP is an active metabolite of 5-FU
High TS levels causes what?
High TS levels → decreased response to 5-FU and antifolates,
increased overall mortality, increased risk of recurrence and progression of cancerAntifolates: MTX, pemetrexed
With High TS levels, can use alternates in chemo regimen such as paclitaxel (taxane) or etoposide (plant alkaloid)
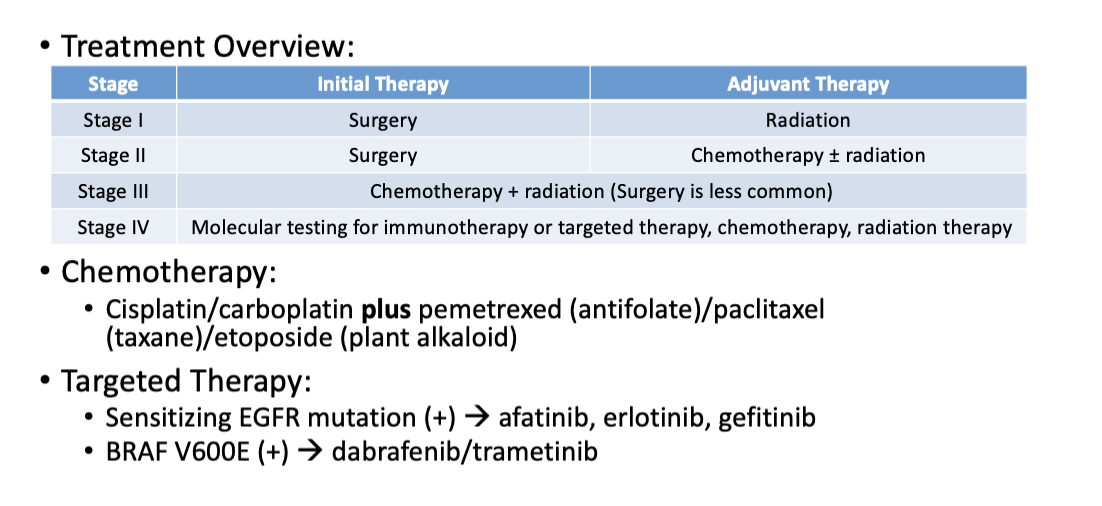
What is the summary of biomarkers with drugs affected?
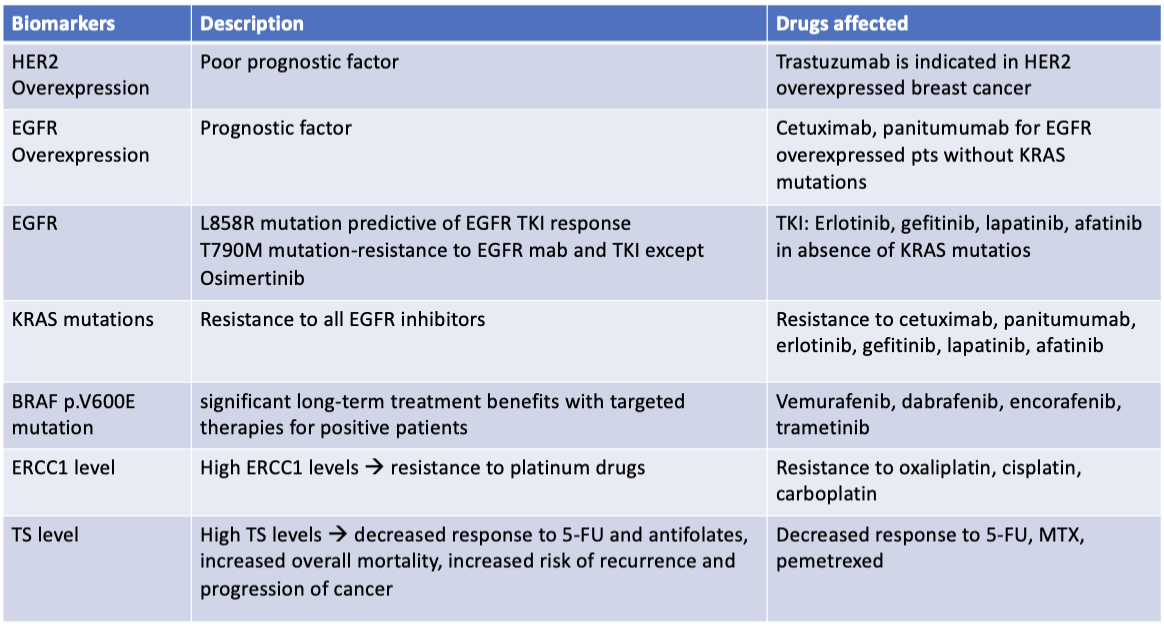
Continuation
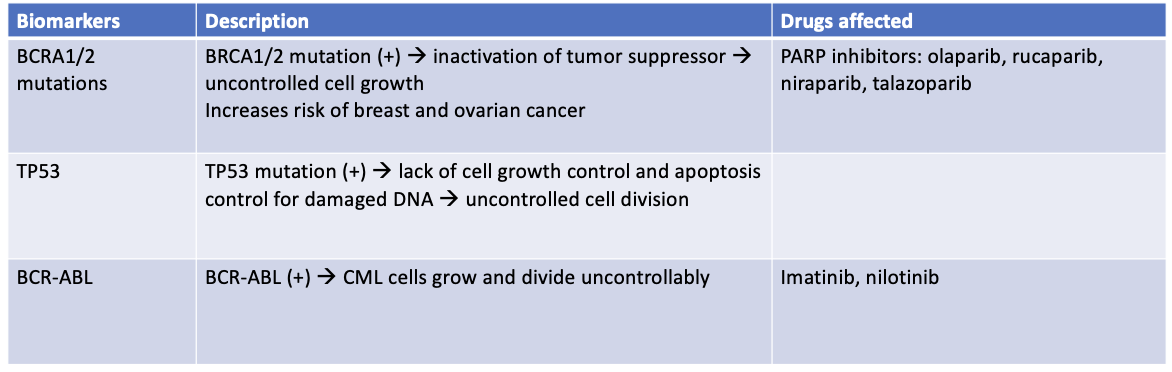
What is the classification of lung cancer?
Lung cancer classification:
Non-Small Cell Lung Cancer (NSCLC) – 85%
Adenocarcinoma, squamous cell carcinoma, large cell carcinoma
Small Cell Lung Cancer (SCLC) – 15%
What is the staging of NSCLC?
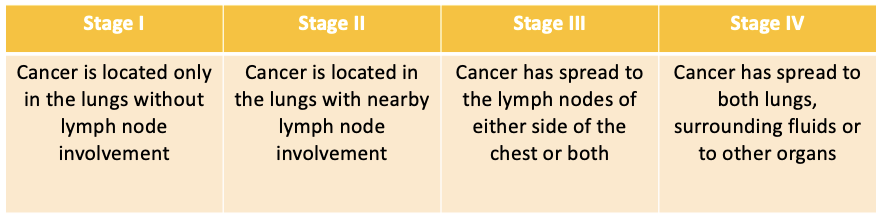
What is the treatment for NSCLC?