W14: ALDEHYDES AND KETONES
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
Both ____________ and ___________ contain a carbonyl functional group.
1.) aldehydes
2.) ketones
Functional group with carbon atom double-bonded to an oxygen atom.
carbonyl group
What is the structural representation for carbonyl group:
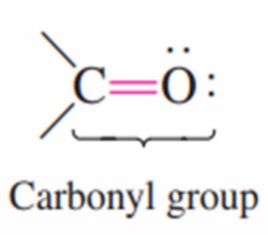
In an aldehyde, one of the two additional bonds that the carbonyl carbon atom forms must be to _________ atom.
hydrogen
In a ketone, both of the additional bonds of the carbonyl carbon atom must be to another _____________ that is part of an alkyl, cycloalkyl, or aromatic group.
carbon atom
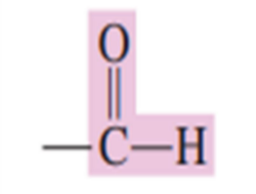
An _____________ is a carbonyl- containing organic compound in which the carbonyl carbon atom has at least one hydrogen atom directly attached to it.
aldehyde
Draw the aldehyde functional group:
A _________ is a carbonyl- containing organic compound in which the carbonyl carbon atom has two other carbon atoms directly attached to it.
ketone
Draw the ketone functional group:
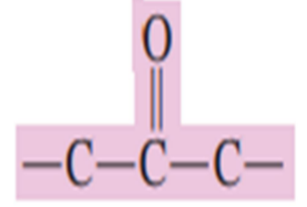
Cyclic ___________ are not possible.
aldehydes
Unlike aldehydes, __________ can form cyclic structures,
ketones
Nomenclature for aldehydes: Name the parent chain by changing the -e ending
of the corresponding alkane name to ___.
-al
Nomenclature for ketones: Name the parent chain by changing the -e ending of the
corresponding alkane name to _____.
-one
______________ is the simplest aromatic ketone.
Acetophenone
The simplest aldehyde, with only one carbon atom, is manufactured on a large scale by the oxidation of methanol.
Formaldehyde
What is the major use of formaldehyde?
manufacture of polymers
At room temperature and pressure, formaldehyde is an ____________.
irritating gas
Bubbling this gas (formaldehyde) through water produces ___________, an aqueous solution containing 37% formaldehyde by mass or 40% by volume.
formalin
Formalin is used for preserving biological specimens, anyone who has experience in a biology laboratory is familiar with the _______ odor of formalin.
pungent
Formalin is also the most widely used ________________ in embalming fluids used by morticians.
Its mode of action involves reaction with protein molecules in a manner that links
the protein molecules together; the result is a “hardening” of the protein.
preservative chemical
a colorless, volatile liquid with a pleasant, mildly “sweet” odor, is the simplest ketone and is also the ketone used in largest volume in industry.
Acetone
Acetone is an _______________ because it is miscible with both water and nonpolar solvents.
excellent solvent
Acetone is the main ingredient in ___________________ that are designed to solubilize water in the gas tank and allow it to pass through the engine in miscible form.
gasoline treatments
Acetone can also be used to ___________ from glassware in the laboratory.
remove water
Small amounts of acetone are produced in the human body in reactions related to obtaining ____________________.
energy from fats
____________ people produce larger amounts of acetone, not all of which can be degraded.
Diabetic
The ________________ in urine is a sign of diabetes.
presence of acetone
In severe diabetes, the odor of acetone can be detected on the person’s __________.
breath
Aldehydes and ketones occur widely in _________.
nature
________________________________, usually have pleasant odors and flavors and are often used for these properties in consumer products such as perfume and fresheners.
Naturally occurring aldehyde and ketones
What are the three important ketone steroid hormones?
1.) Testosterone
2.) Progesterone
3.) Cortisone
the hormone that controls the development of male sex characteristics
testosterone
the hormone secreted at the time of ovulation in females
progesterone
a hormone from the adrenal glands that is used medicinally to relieve inflammation
cortisone
The ____ and ____ aldehydes are gases at room temperature.
1.) C1
2.) C2
The ____________________ straight-chain saturated aldehydes are liquids, and the higher aldehydes are solids.
C3 through C11
The presence of ____________ tends to lower both boiling points and melting points, as does the presence of unsaturation in the carbon chain.
alkyl groups
colorless liquids at room temperature
Lower-molecular-mass ketones
Aldehydes and ketones have lower boiling points than the _________________ because no hydrogen bonding occurs as it does with alcohols.
corresponding alcohols
Aldehydes and ketones have higher boiling points than _______ because of dipole–dipole attractions between molecules.
alkanes
As the hydrocarbon portions get larger, the water solubility of aldehydes and ketones __________.
decreases
Aldehydes and ketones can be produced by the ________ of primary and secondary alcohols.
oxidation
__________ do not undergo the further oxidation that aldehydes do.
Ketones