Memorization tasks Ochem1
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
pKa for CH3 → CH4
-50
pKa for CH3 ← CH4
+50
pKa to add an H to a strong acid
-0
pKa to remove an H from a strong acid
+0
pKa for H → H2
-40
pKa for H ← H2
+40
pKa for NR2 → NR2H
-35
pKa for NR2 ← NR2H
+35
pKa for RO → ROH
-16
pKa for RO ← ROH
+16
pKa for R3N → R3NH
-9
pKa for R3N ← R3NH
+9
pKa for F → FH
-3
pKa for F ← FH
+3
pKa for Cl → HCl
-0
pKa for Cl ← HCl
+0
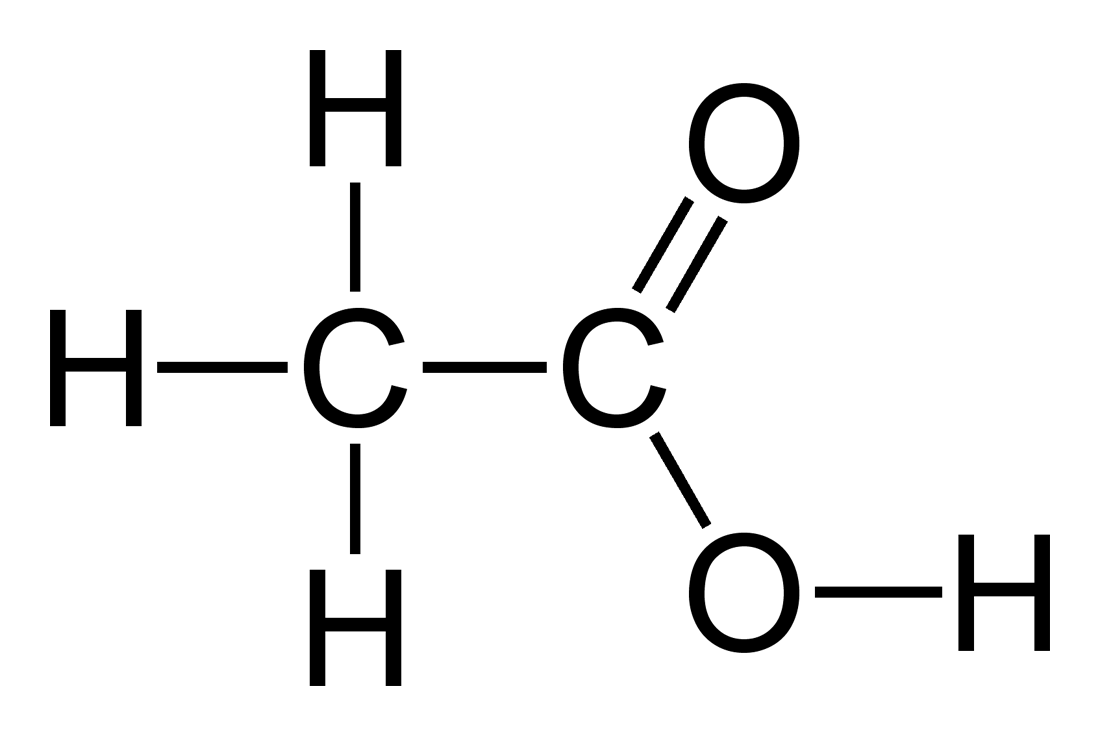
pKa for COOH → COO-
+5
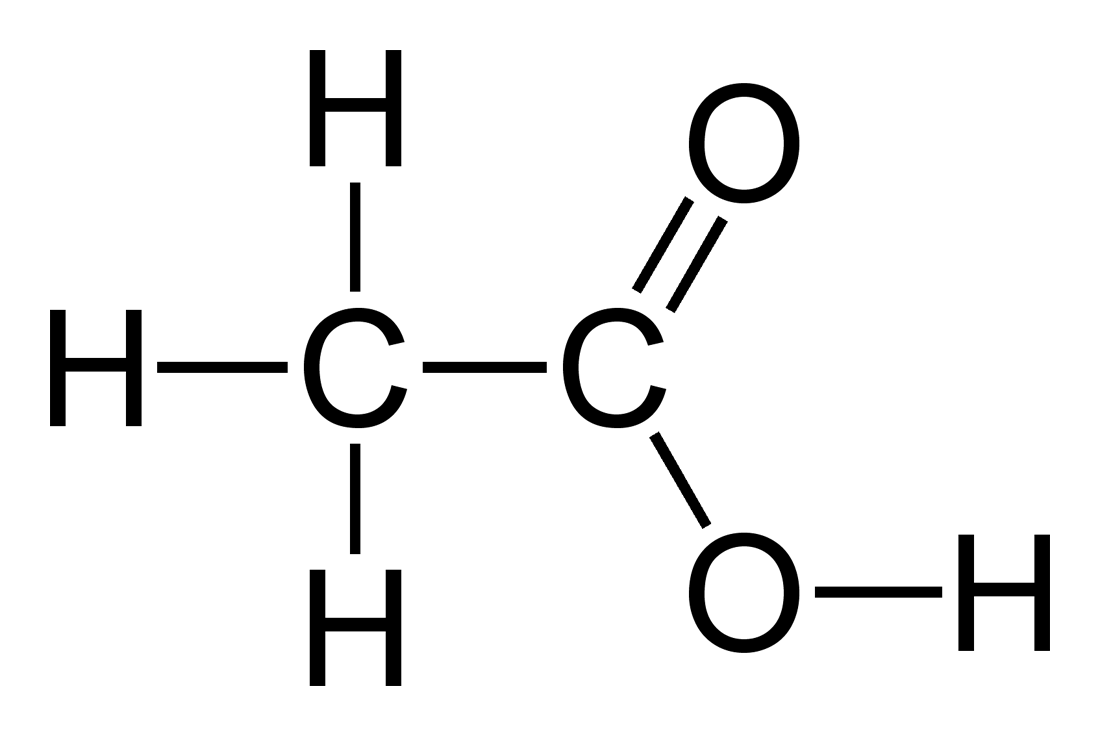
pKa for COOH ← COO-
-5
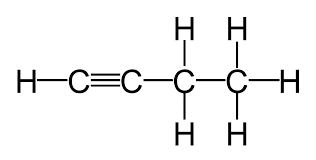
pKa for RCCH → RCC-
+25
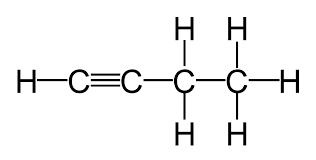
pKa for RCCH ← RCC-
-25
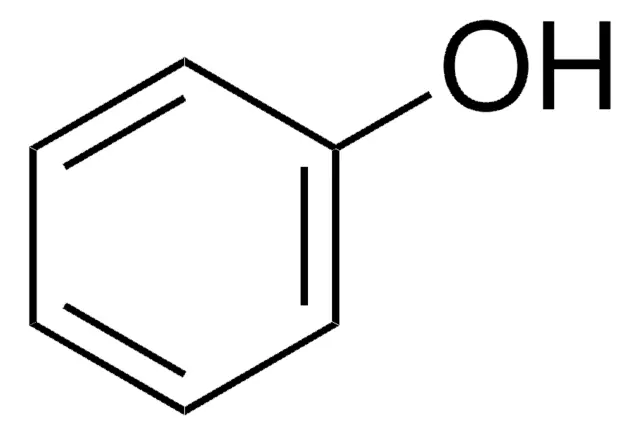
pKa for C6H5OH → C6H5O-
+10
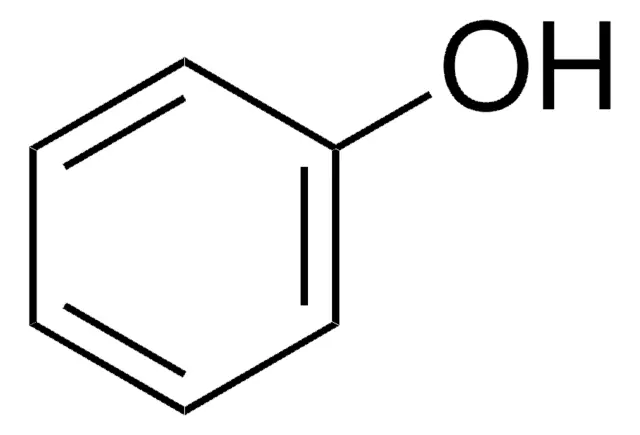
pKa for C6H5OH ← C6H5O-
-10
1 is
meth
2 is
eth
3 is
prop
4 is
but
5 is
pent
6 is
hex
7 is
hept
8 is
oct
9 is
non
10 is
dec
-CH3 is
methyl
-CH2CH3 is
ethyl
-CH2CH2CH3 is
propyl
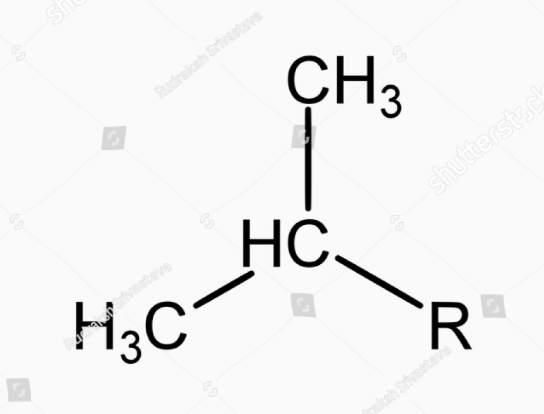
this is
isopropyl (i-Pr)
-CH2CH2CH2CH3 is
butyl
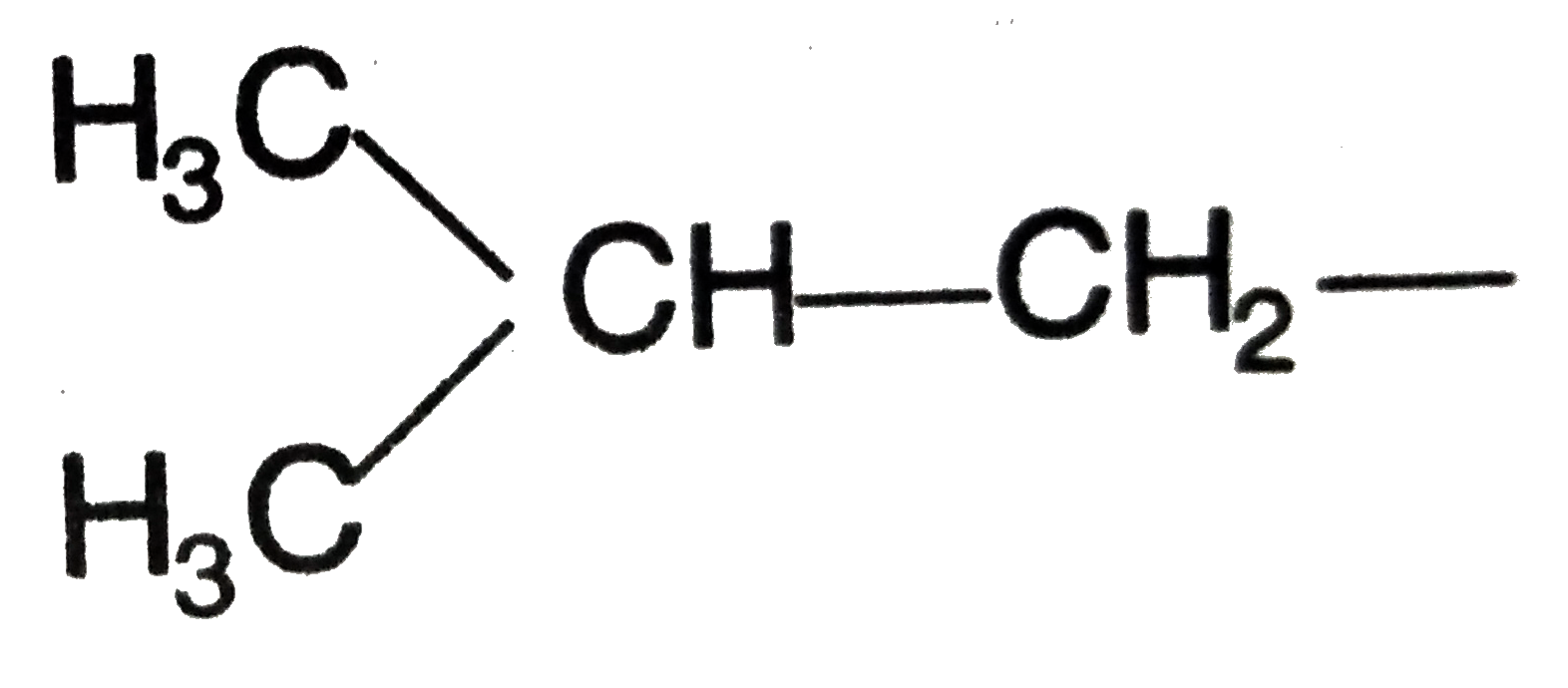
this is
isobutyl
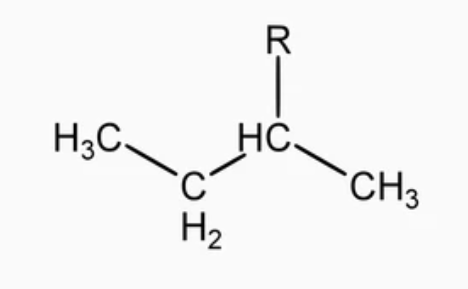
this is
secbutyl
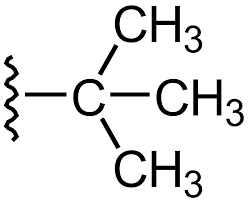
This is
tertbutyl
-CH2CH2CH2CH2CH3 is
pentyl