A2 Chemistry topic 6: Carboxylic acid derivatives, nitrogen-containing compounds and polymers
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
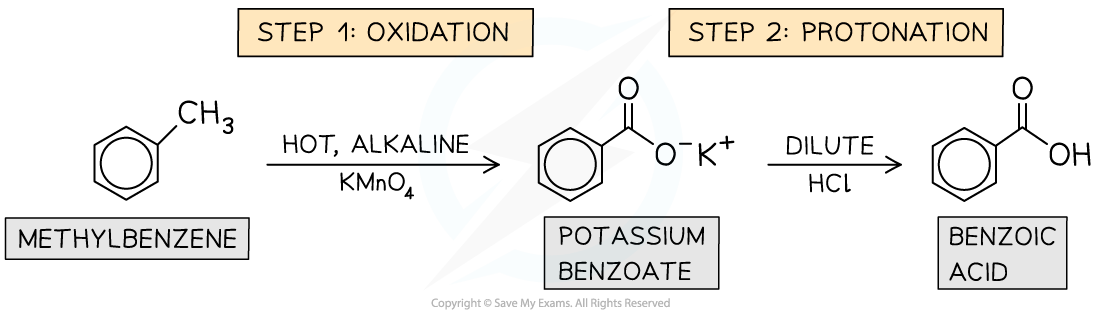
produced by the oxidation of alkylbenzenes
the alkyl side-chain in alkylbenzenes, e.g. methylbenzene, can be oxidised to a carboxylic acid
alkylbenzene is heated under reflux with a solution of hot alkaline KMnO4 (this is the oxidising agent)
purple colour disappears and forms a brown ppt (from the formation of MnO2) as Mn7+ reduces to Mn4+
the mixture is then acidified with dilute acid (e.g. HCl) to protonate the organic product form and produce benzoic acid
how is benzoic acid produced
liquid PCl3 and heat
solid PCl5
liquid SOCl2
what are the three ways in which an acyl chloride can be formed from a carboxylic acid
3CH3CO2H (l) + PCl3 (l) → 3CH3COCl (l) + H3PO3 (l)
describe the reaction for the formation of an acyl chloride from a carboxylic acid using PCl3 and heat, using ethanoic acid as an example
CH3CO2H (l) + PCl5 (s) → CH3COCl (l) + POCl3 (l) + HCl (g)
HCl gas produces steamy white fumes
describe the reaction for the formation of an acyl chloride from a carboxylic acid using PCl5, using ethanoic acid as an example
CH3CO2H (l) + SOCl2 (l) → CH3COCl (l) + SO2 (g) + HCl (g)
describe the reaction for the formation of an acyl chloride from a carboxylic acid using SOCl2, using ethanoic acid as an example
methanoic acid is a strong reducing agent and gets further oxidised to form CO2
this occurs by warming methanoic acid with mild oxidising agent such as Fehling’s or Tollens’ reagent
Fehling’s: Cu2+ reduced to Cu+, forms red ppt - Cu2O
Tollens’: Ag+ reduced to Ag,
stronger oxidising agents such as acidified KMnO4 or K2Cr2O7 can also be used
purple KMnO4 solution turns colourless, Mn7+ ions reduced to Mn2+
orange K2Cr2O7 solution turns green, Cr6+ ions reduced to Cr3+
describe how carboxylic acids, such as methanoic acid, can be further oxidised
use strong oxidising agent such as warm acidified KMnO4, forms CO2
describe how carboxylic acids, such as ethanedioic acid, can be further oxidised
electrons in the O-H bond are drawn towards the C-O bond
the electrons in the C-O bond are drawn towards the C=O bond
overall, the O-H bond is weakened due to the carbonyl group removing electron density from it and drawing it towards itself
carboxylic acids can therefore more easily lose a proton compared to the phenols and alcohols which lack this electron-withdrawing group, therefore carboxylic acids are stronger acids than alcohols and phenols
describe how the strength of the O-H bond explains the acidity of a carboxylic acid relative to alcohols and phenols
conjugate base of carboxylic acids is the carboxylate ion
the charge density on the oxygen atom is spread out over the carboxylate ion
this is because charge is delocalised on the electronegative carbonyl oxygen atom
as a result, the electrons on the oxygen atom are less available for bond formation with an H+ ion to reform the undissociated acid molecule with -COOH group
the position of the dissociation equilibrium lies more to the right compared to alcohols and phenols
describe how the stability of the carboxylate ion explains the acidity of a carboxylic acid relative to alcohols and phenols
conjugate base of alcohols is the alkoxide ion
the alkyl group in the ion is an electron-donating group that donates electron density to the oxygen atom
as a result, the electron density on the oxygen atom is more readily available for bond formation with an H+ ion
alkoxide ions also lack the ability to delocalise the charge density on the entire ion
therefore alkoxides are less stable than the alcohol itself so is more likely to reform the alcohol
alcohols are therefore weaker acids than carboxylic acids and phenols
dissociation equilibrium position lies to the left
describe how the stability of the alkoxide ion explains the acidity of a carboxylic acid relative to alcohols and phenols
charge density on the oxygen atom is spread out across the entire ion
this delocalisation stabilises the phenoxide ion
as a result, the electrons on the oxygen atom are less available for bond formation with a proton (H+ ion)
conjugate base of phenols are therefore more stable than the phenol
however, since the delocalisation of charge density is on the carbon atoms and not on electronegative oxygen atoms like in the carboxylate ion, phenoxide ions are less stable than carboxylate ions
therefore phenols are weaker acids than carboxylic acids
position of dissociation equilibrium lies more to the left than carboxylic acids and more to the right than alcohols
describe how the stability of phenoxide ions explains the acidity of a carboxylic acid relative to alcohols and phenols
chlorine atoms act as electron-withdrawing groups that make the carboxylic acid stronger
the O-H bond is further weakened as the chlorine atom draws even more electron density away from this bond
this further extends the delocalisation of the negative charge on the -COO- group of the carboxylate ion henceforth making it more stable and less likely to bond with the H+ ion
the more electron-withdrawing groups there are on the carbon attached to the -COOH group, the stronger the acid
on the contrary, carboxylic acids have an electron-donating methyl group which strengthens the O-H bond, the methyl group donates negative charge towards the -COO- group which becomes more likely to accept the H+ ion
describe and explain the relative acidities of chlorine-substituted carboxylic acids
acyl chloride + alcohol → ester
ethanoyl chloride + ethanol → ethyl ethanoate + HCl
benzoyl chloride + phenol → phenyl benzoate + HCl
describe the reaction of alcohols and acyl chlorides to form esters, using ethyl ethanoate and phenol benzoate as examples

results in the formation of a carboxylic acid and an HCl molecule
this is an addition-elimination reaction
water molecule is added across the C=O bond
HCl molecule is eliminated
describe the hydrolysis of acyl chlorides
acyl chlorides reacts with alcohols and phenols to form esters
esters can also be formed from carboxylic acids reacting with alcohols but this reaction is slower as carboxylic acids are less reactive than acyl chlorides and therefore the reaction does not go to completion (so less product is formed)
esterification of acyl chlorides is an addition-elimination reaction
alcohol/phenol adds across the C=O bond
HCl molecule is eliminated
describe the process in which ester is formed from an acyl chloride
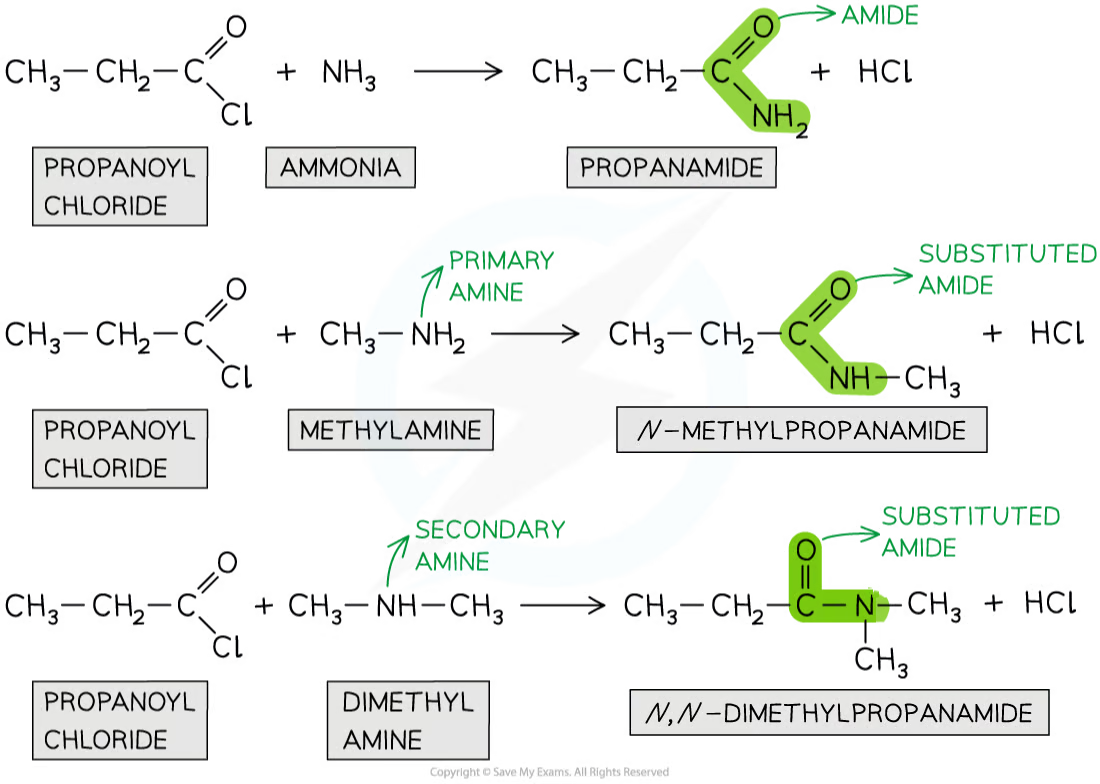
acyl chlorides can form amides from their condensation reactions with amines and ammonia
nitrogen atom in ammonia and amines has a lone pair of electrons which attacks the carbonyl carbon atom in the acyl chloride
product is a non-substituted amide (when reacted with ammonia) or substituted amide (when reacted with a primary and secondary amine)
this is an example of an addition-elimination reaction
the amine or ammonia molecule adds across the C=O bond
HCl molecule is eliminated
describe the formation of amides from acyl chlorides
addition of a nucleophile across the C=O bond
elimination of a small molecule such as HCl or H2O
describe the general mechanism of addition-elimination reactions
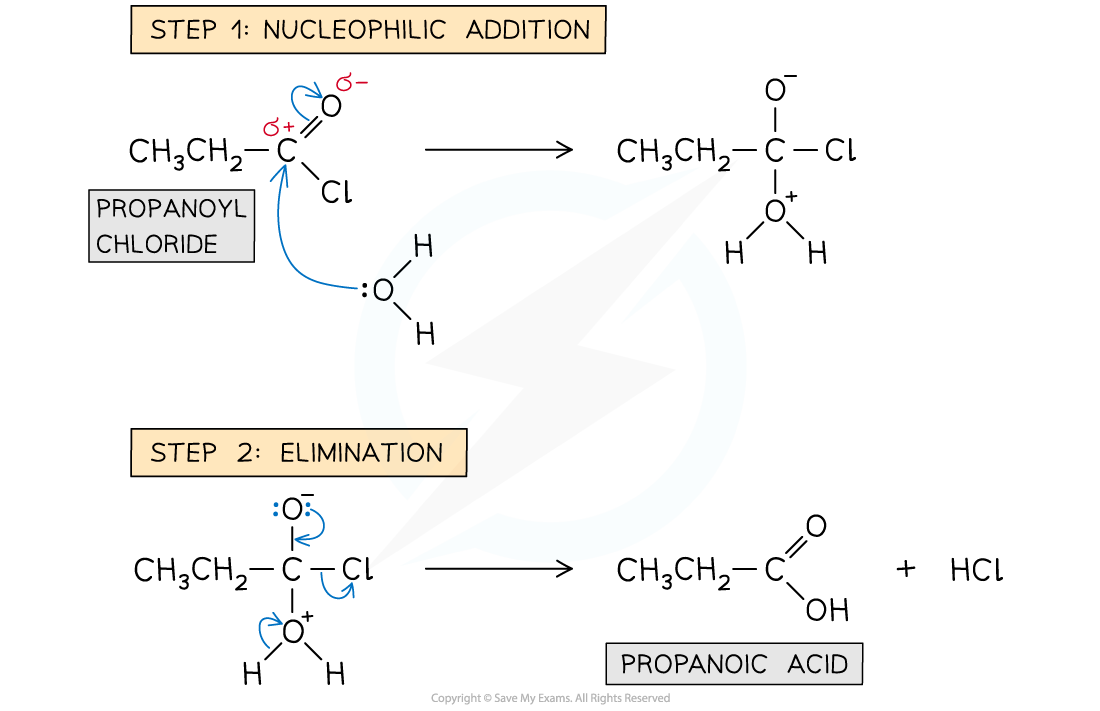
water molecule acts as nucleophile
lone pair of electrons on oxygen atom initiate attack on the carbonyl carbon atom
this is followed by the elimination of an HCl molecule
describe the mechanism of hydrolysis of acyl chlorides

alcohols or phenols act as a nucleophile
the lone pair of electrons on the oxygen atoms initiate an attack on the carbonyl atom
this is followed by the elimination of an HCl molecule
with phenols, reaction requires heat and presence of a base for the reaction to proceed
the base deprotonates the phenol to form a phenoxide ion which is a better nucleophile than a phenol molecule
the phenoxide ion initiates attack on the carbonyl carbon atom
small molecule of NaCl is eliminated
describe the reaction mechanism for the formation of an ester from an acyl chloride
nitrogen in ammonia and primary/secondary amines act as a nucleophile
lone pair on the nitrogen atom initiate an attack on the carbonyl carbon atom
HCl molecule is eliminated
both reactions of acyl chlorides with ammonia and amines are vigorous but have differences
reaction with ammonia produces a non-substituted amide and white fumes of HCl are formed
reaction with amines produces a substituted amide and the HCl formed reacts with the unreacted amine to form a white organic ammonium salt
describe the reaction mechanism for the formation of amides from acyl chlorides
acyl chloride>alkyl chloride>aryl chloride
what is the relative ease of hydrolysis of acyl chlorides, alkyl chlorides, and halogenoarenes
acyl chlorides are most readily hydrolysed at room temp.
this is because the carbon bonded to the chlorine atom is also bonded to an oxygen atom
There are two strong electronegative atoms pulling electrons away from the carbonyl carbon, leaving it very δ+
C-Cl bond is therefore weakened and nucleophilic attack of the carbonyl carbon is much more rapid
the carbonyl carbon in alkyl chlorides is only attached to one electronegative atom which pulls electrons from it
this carbon atom is therefore not very δ+ and the C-Cl bond is stronger than the C-Cl bond in acyl chlorides
hydrolysis of alkyl chlorides therefore requires a strong alkali (such as OH-) to be refluxed with it
an OH- ion will hydrolyse the alkyl chloride as it is a stronger nucleophile than H2O
in aryl chlorides, the carbon atom bonded to the chlorine atom is part of the delocalised π bonding system of the benzene ring
one of the lone pairs of electrons of the Cl atom overlaps with this delocalised system
C-Cl bond therefore has some double-bond character causing it to become stronger
as a result, the C-Cl bond is difficult to break and hydrolysis will not occur
explain the relative ease of hydrolysis of acyl chlorides, alkyl chlorides, and halogenoarenes

nucleophilic substitution reaction occurs where the lone pair on the nitrogen acts as a nucleophile and replaces the halogen in the halogenoalkane
when a halogenoalkane is reacted with excess, hot ethanolic ammonia under pressure, a primary amine is formed
describe how amines can be produced from the reaction of halogenoalkanes with ammonia

nucleophilic substitution
nitrogen on primary amine acts as nucleophile and replaces the halogen on the halogenoalkane
when a halogenoalkane is reacted with a primary amine in ethanol and heated in a sealed tube, under pressure a secondary amine
describe how secondary amines can be produced the reaction of halogenoalkanes with primary amines
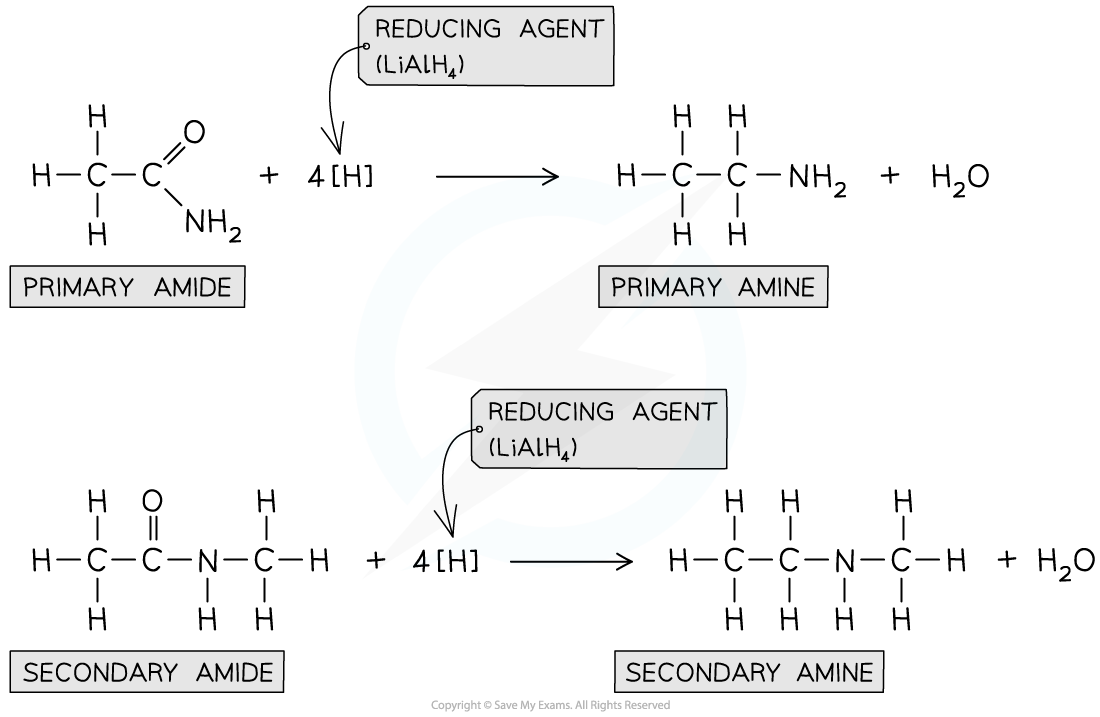
amines can be formed from the reduction of amides by LiAlH4 in dry ether
whether a primary or secondary amine is formed depends on the nature of the amide
describe how amines are produced from the reduction of amides
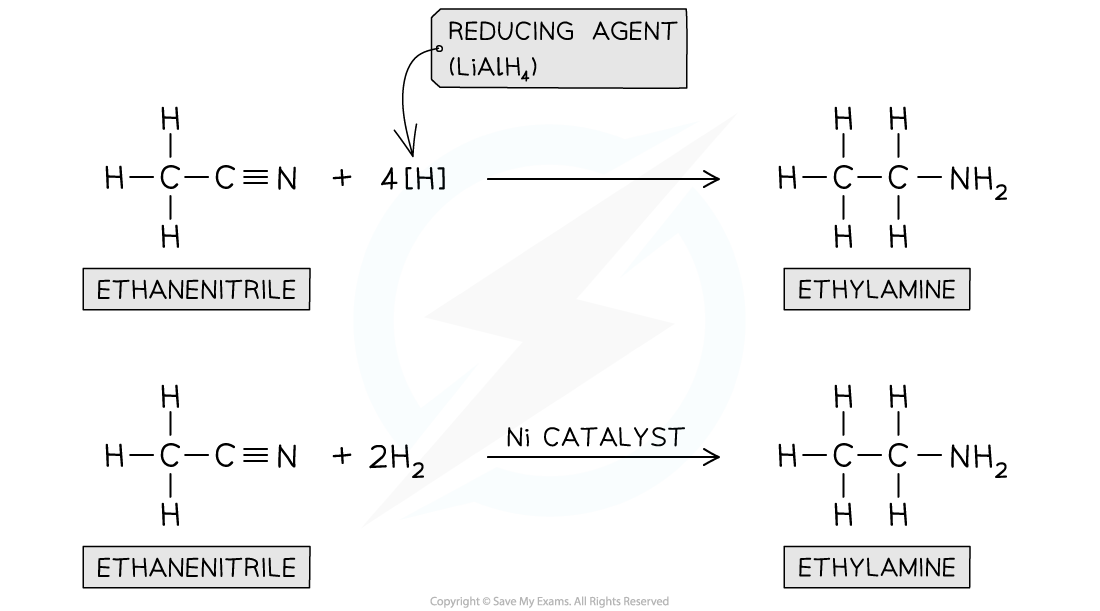
nitriles contain a -CN functional group that can be reduced to an -NH2 group
the nitrile vapour and hydrogen gas are passed over a nickel catalyst or LiAlH4 in dry ether can be used to form a primary amine
describe how amines are produced from the reduction of nitriles
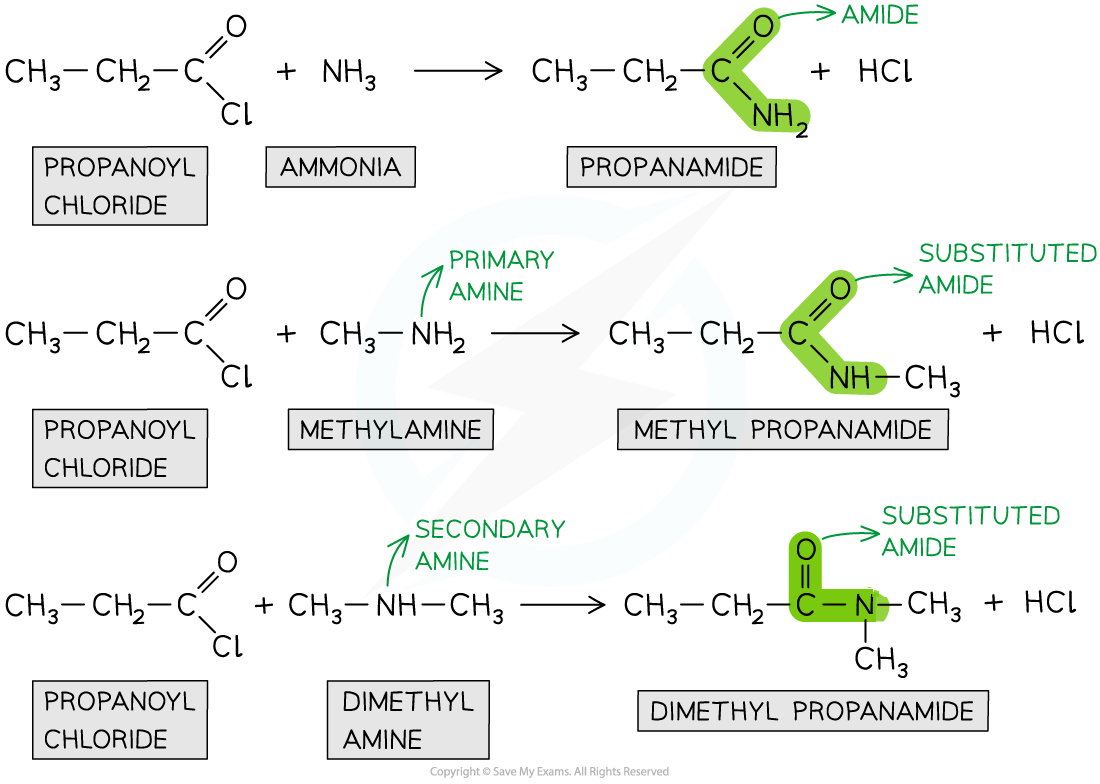
the chlorine atom in acyl chlorides are electronegative and draw electron density away from the carbonyl carbon
this means the carbonyl carbon becomes electron deficient and therefore susceptible to attack by nucleophiles
the nitrogen atom in ammonia and amines have a lone pair of electrons that can act as a nucleophile and attack the carbonyl carbon
as a result the C-Cl bond is broken and an amide is formed
reaction with primary and secondary amines will give a substituted amide
reaction with ammonia will produce a non-substituted amide
describe how amides are produced from the condensation reaction between an acyl chloride and ammonia or an amine
the strength of basicitiy of an amine depends on the availability of the lone pair of electrons on the nitrogen atom to form a dative covalent bond with a proton
the more readily the lone pair of electrons is available, the stronger the base is
factors that affect the basicity of an amine are:
positive inductive effect - some groups such as alkyl groups donate electron density to the nitrogen atom causing the lone pair of electrons to become more available therefore increasing the amines basicitiy
delocalisation - presence of an aromatic ring such as the benzene ring causes the lone pair of electrons on the nitrogen to be delocalised into the benzene ring
the lone pair becomes less available to form a dative bond with ammonia and hence decreases the amines basicitiy
describe and explain the basicitiy of aqueous solutions of amines
produced in a three-step synthesis reaction followed by the separation of phenylamine from the reaction mixture
benzene undergoes nitration with concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) at 25 degrees to 60 degrees to form nitrobenzene
nitrobenzene is reduced with hot tin (Sn) and concentrated hydrochloric acid (HCl) under reflux to form an acidic reaction mixture that contains the organic product C6H5N+H3
sodium hydroxide (NaOH) is added to the acidic reaction mixture to form phenylamine
the phenylamine is separated from the reaction mixture by steam distillation
describe how phenylamines are prepared

electrophilic substitution reaction
the lone pair of electrons on the nitrogen atom of the phenylamine donates electron density into the benzene ring
the delocalisation of electrons causes an increased electron density in the benzene ring
the benzene ring therefore becomes activated and becomes more readily attacked by electrophiles
the incoming electrophiles are directed to the 2,4, and 6 positions
phenylamine therefore reacts under mild conditions with aqueous bromine at room temperature to form 2,4,6-tribromophenylamine
describe the bromination of phenylamine
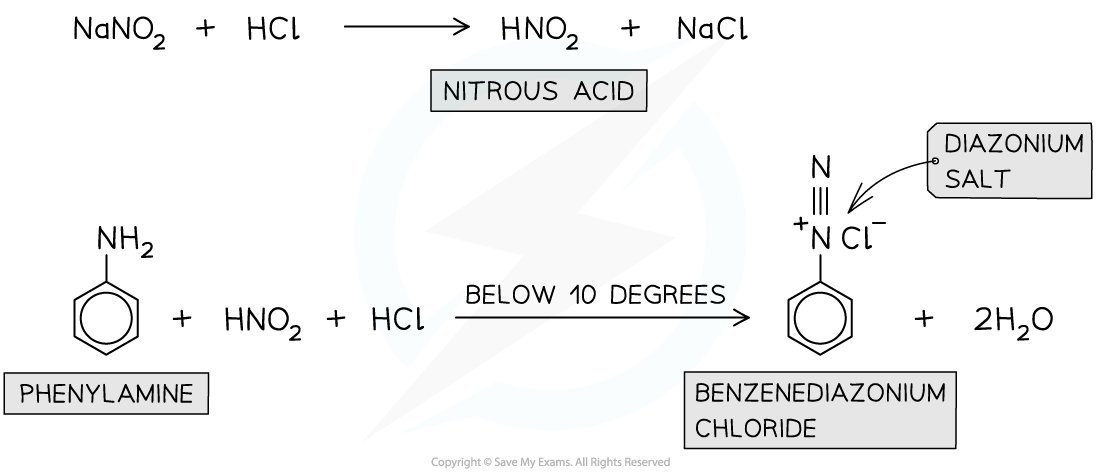
diazonium compounds are very reactive compounds containing an -N2+ group
the amine (-NH2) group of phenylamines will react with nitrous acid (HNO2) at a temperature below 10 degrees to form diazonium salts
since nitrous acid is unstable, it has to be made in a test tube by reacting sodium nitrite (NaNO2) and dilute acid (such as HCl)
the diazonium salts are so unstable that upon further warming with water they will form phenol
describe the reaction of the formation of diazonium salt from phenylamine
ethylamine>ammonia>phenylamine
ethylamine
electron-donating alkyl groups has a positive inductive effect by donating electron density to the nitrogen atom causing its lone pair of electrons to become more available to form a dative covalent bond with a proton
ammonia
no electron-donating group hence it is a weaker base than ethylamine
more basic than phenylamine as there are no aromatic rings to cause the delocalisation of nitrogen’s lone pair of electrons
phenylamine
lone pair of electrons overlap with the conjugated system on the benzene ring and becomes delocalised; as a result, the lone pair of electrons become less readily available to form a covalent dative bond with a proton
describe and explain the relative basicity of aqueous ammonia, ethylamine, and phenylamine
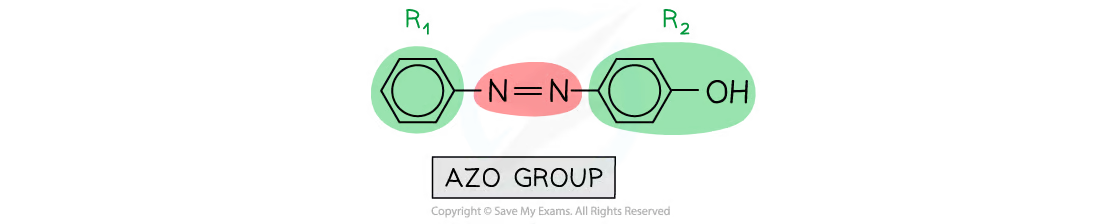
organic compounds that have an R1-N=N-R2
they are often used as dyes and are formed in a coupling reaction between the diazonium ion and an alkaline solution of phenol
what are azo compounds
formation of nitrous acid
nitrous acid is very unstable so has to made in a test tube using sodium nitrate (NaNO2) and hydrochloric acid (HCl)
diazotization
the reaction between nitrous acid and phenylamine to form the diazonium ion is called diazotization
reaction mixture must be kept under 10 degrees using ice otherwise the diazonium ion will thermally decompose to benzene and nitrogen (N2)
requires dilute acid (e.g. HCl)
coupling reaction
the diazonium ion acts as an electrophile and substitutes into the benzene ring of the phenol at the 4th position
alkaline conditions are required to deprotonate the organic product and form the azo compound
describe the coupling of benzenediazonium chloride with phenol in NaOH(aq) to form an azo compound
the delocalised electrons in the π bonding systems of the two benzene rings are extended through the -N=N- which acts as a bridge between the two rings
as a result of the delocalisation of electrons throughout the compound, azo compounds are very stable
why are azo compounds very stable
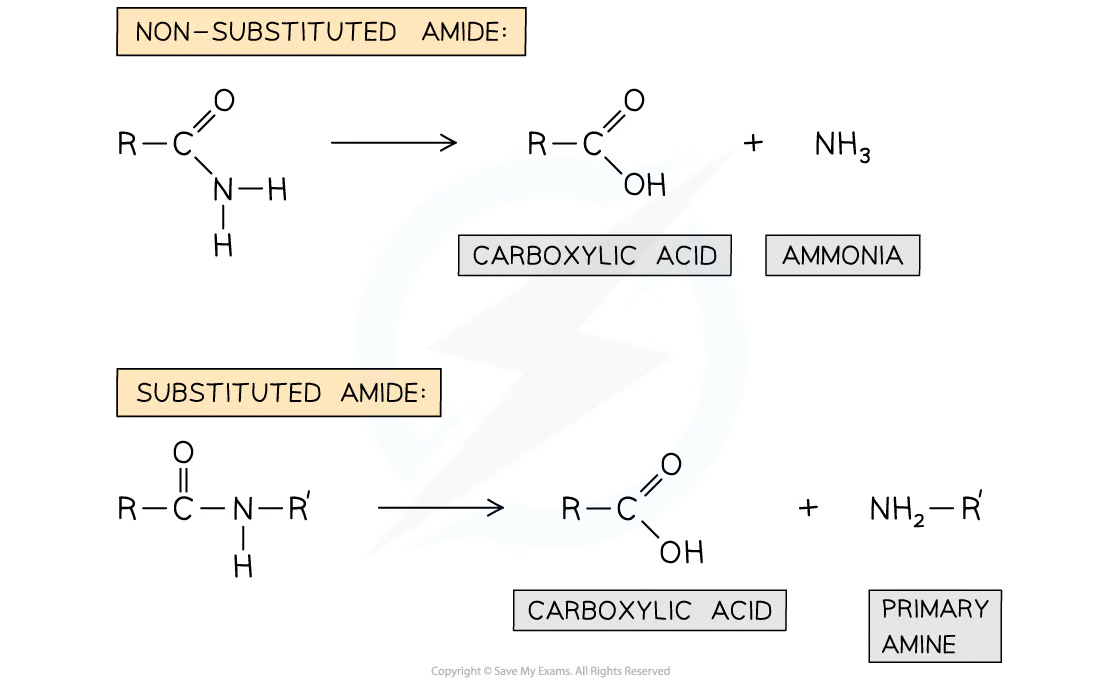
the amide link can be broken down by hydrolysis by refluxing with aqueous alkali or acid
the products of a non-substituted amide:
carboxylic acid
ammonia
the products of a substituted amide:
carboxylic acid
primary amine
ammonia will react in excess acid to form an ammonium salt
carboxylic acid will get deprotonated in excess base to form a carboxylate ion
describe the hydrolysis of amides with aqueous alkali or acid
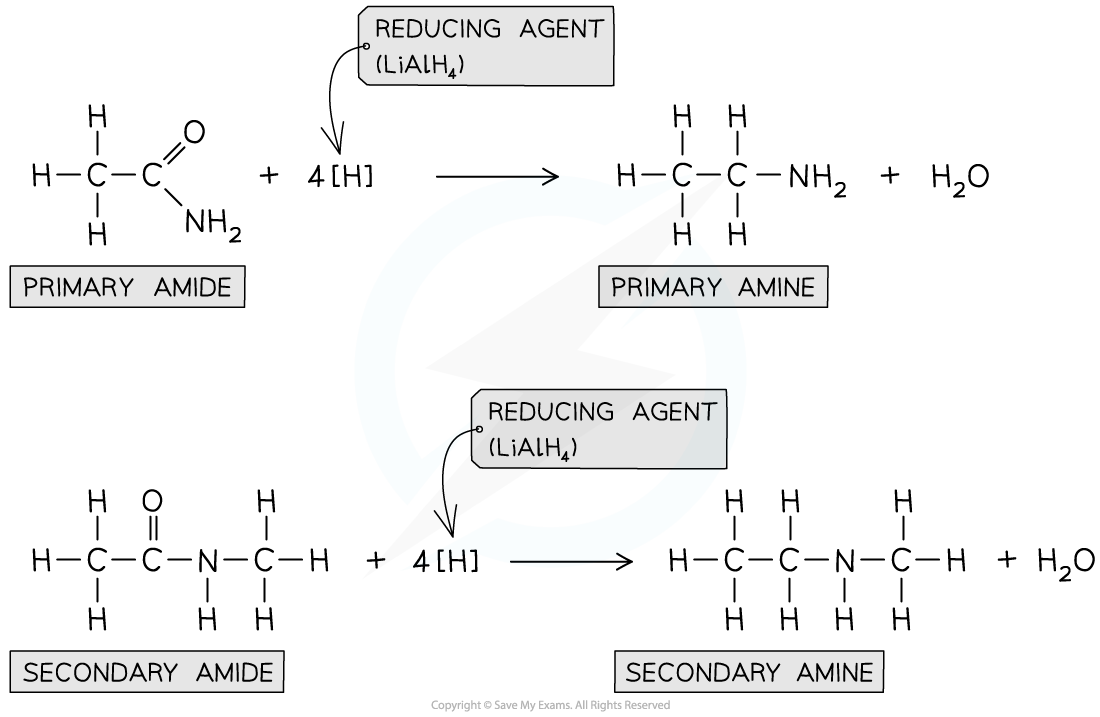
the C=O group in amides can be reduced by the strong reducing agent LiAlH4
the product of a non-substituted amide are:
primary amine
water
the products of a substituted amide are:
secondary amine
water
describe the reduction of amides with LiAlH4 to form an amine
due to the presence of the electron-withdrawing oxygen atom in the amide group, electron density is removed from the nitrogen atom
the lone pair on the nitrogen atom, therefore, becomes less readily available and is not available to donate to an electron-deficient species
since this electron-withdrawing oxygen is characteristic of amides and not amines, amides are much weaker bases than amines
why are amides much weaker bases than amines
organic compounds that contain the two functional groups:
a basic amino (-NH2) group
an acidic carboxylic acid (-COOH) group
what are amino acids
due to the presence of both a basic and acidic group, they are said to be amphoteric meaning they can act as both acids and bases
why are amino acids said to be amphoteric
an ion with both a positive and negative charge
what is a zwitterion

amino acids interact intramolecularly within themselves to form a zwitterion
because of these charges in a zwitterion, there are strong intermolecular forces of attraction between amino acids
amino acids are therefore soluble crystalline solids
describe how amino acids interact within themselves to form a zwitterion and how it affects the properties of an amino acid
a solution of amino acids in water will exist as zwitterions with both acidic and basic properties
they act as a buffer solution as they resist change in pH when small amounts of acid or alkali are added
if an acid is added (and pH is lowered)
the -COO- part of the zwitterion will accept an H+ ion to reform the -COOH group
this causes the zwitterion to become a positively charged ion
if base is added (and pH is raised)
the -NH3+ part of the zwitterion will donate an H+ ion to reform the -NH2 group
this causes the zwitterion to become negatively charged
the pH can be slightly adjusted to reach a point where neither the negatively charged or positively charged ions dominate and the amino acid exists as a neutral zwitterion
this is called the isoelectric point of the amino acid
describe what the isoelectric point is
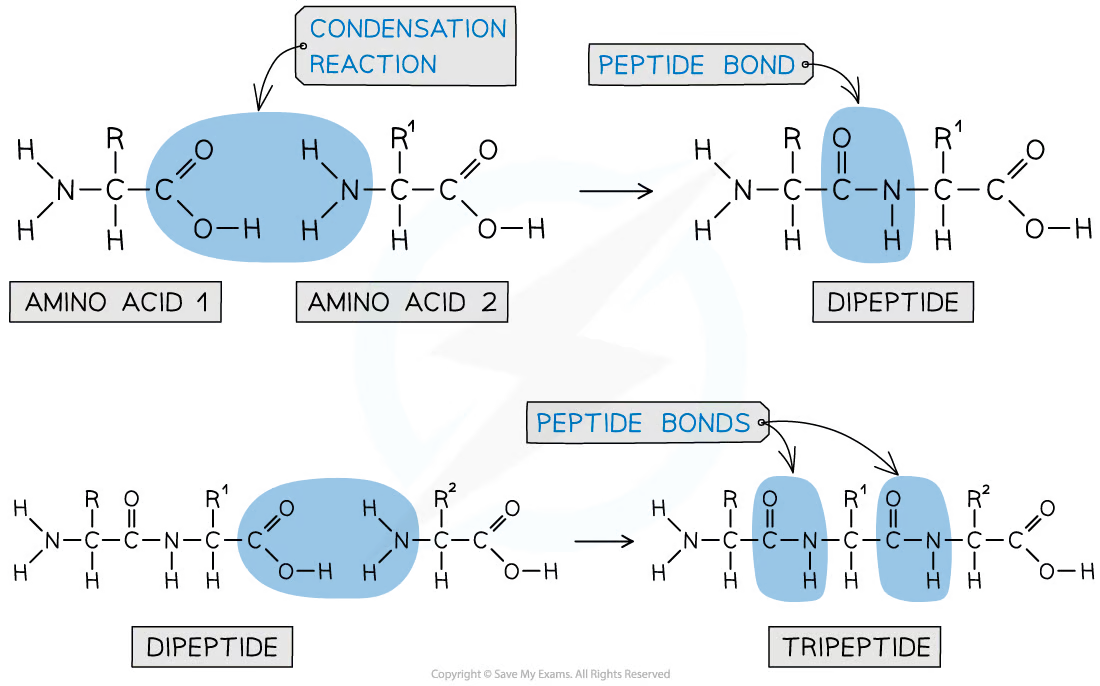
the -NH2 group of one amino acid can react with the -COOH group of another amino acid in a condensation reaction to form a dipeptide
the new bond form between the two amino acids is called a peptide link or peptide bond
since this is a condensation reaction, a small molecule (H2O) is removed
the dipeptide still contains an -NH2 and -COOH group at each end of the molecule so can therefore undergo another condensation reaction to form a tripeptide
describe the formation of peptide bonds
analytical technique used in biochemical analysis to identify and purify proteins by separating ions by placing them in an electric field
what is electropheresis
sample of amino acids is placed between two oppositely charged electrodes
the positively charged ions will move towards the negatively charged electrode
the negatively charged ions will move towards the positively charged electrode
the rate (how fast) at which ions move towards electrodes depends on:
the size of the ions: larger ions move more slowly
the charge of the ions: highly charged ions move more quickly
An electropherogram is the series of bands which are observed on the paper or gel after electrophoresis has occurred
Each band in the electropherogram corresponds to a particular species
describe how electropheresis works
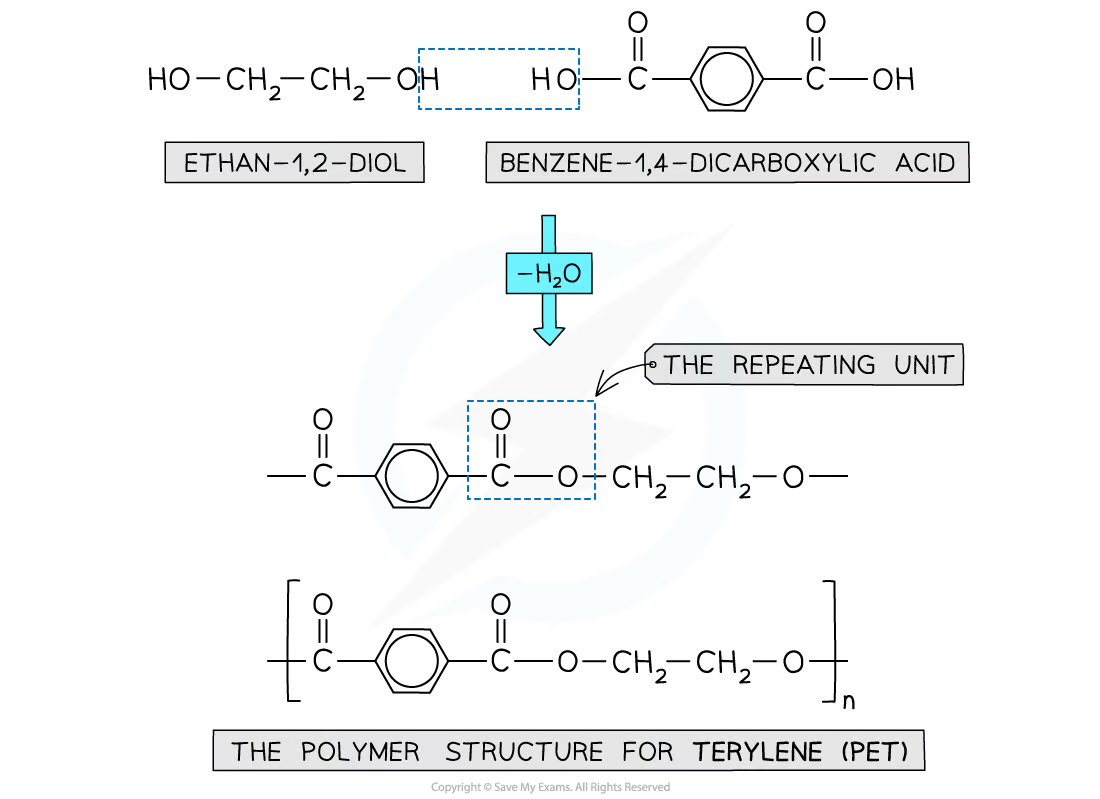
occurs via the condensation reaction between a diol and a dicarboxylic acid
a diol contains 2 -OH groups
a dicarboxylic acid contains 2 -COOH groups
forms an ester bond
when the polyester is formed, one of the -OH groups on the diol and the hydrogen atom of the -COOH are expelled as a water molecule (H2O)
the resulting polymer is a polyester
describe the formation of polyesters via condensation between a diol and a dicarboxylic acid
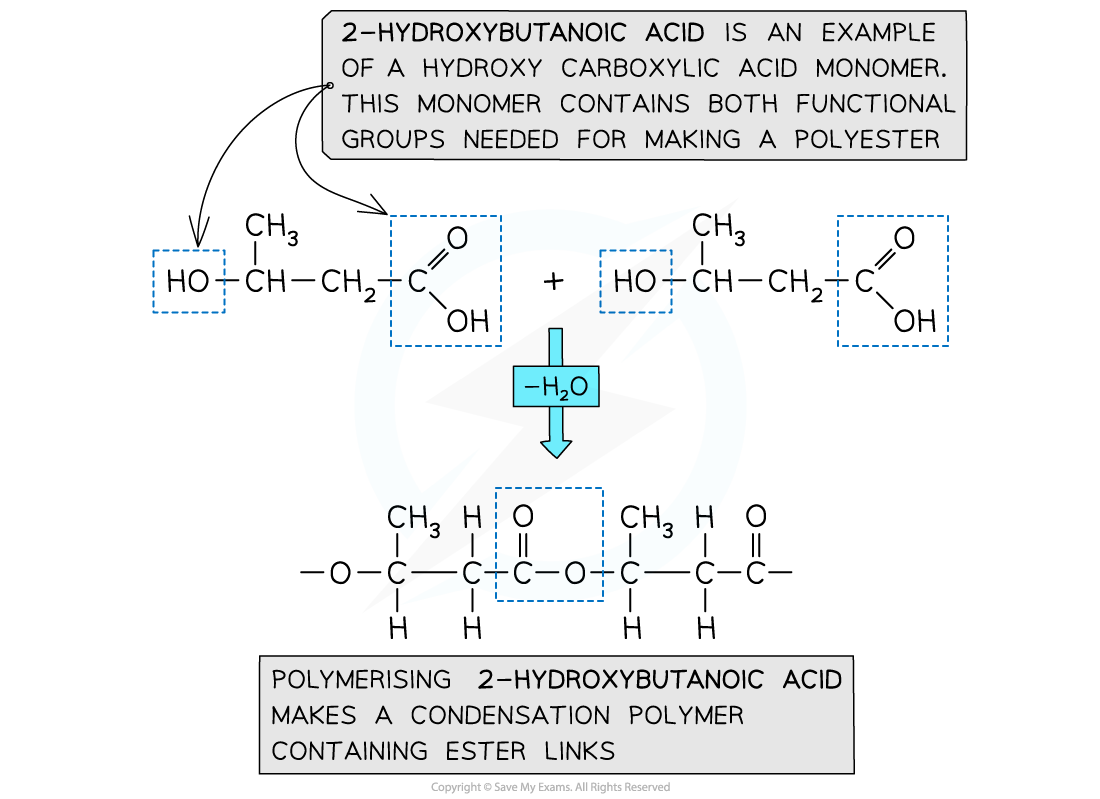
hydroxycarboxylic acids contain an alcohol group at one end of the molecule and a carboxylic acid at the other
molecules undergo a condensation reaction to form a polyester, expelling water as a small molecule
forms ester bond
describe the formation of a polyester via condensation reaction between two molecules of hydroxycarboxylic acids
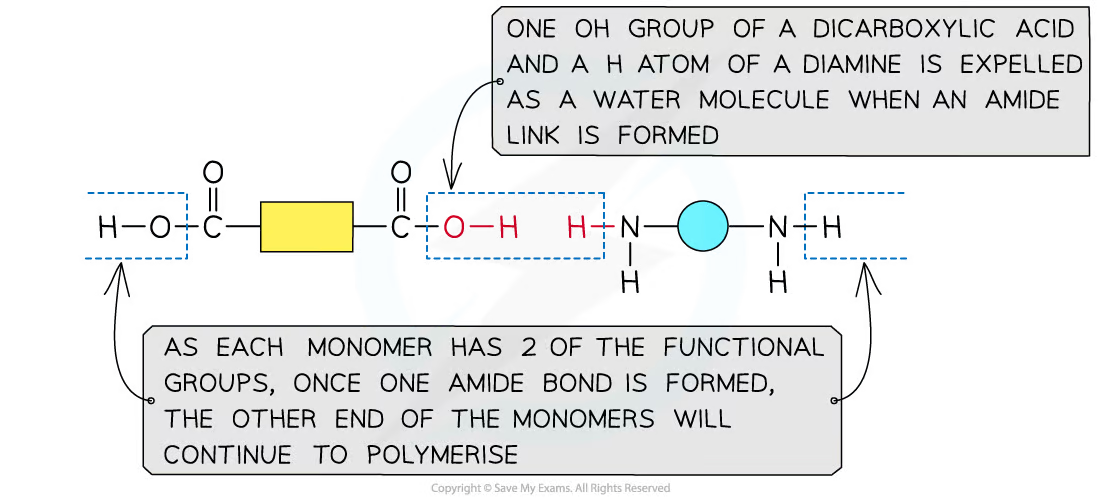
a diamine and dicarboxylic acid is required to make a polyamide
a diamine contains 2 -NH2 groups
a dicarboxylic acid contains 2 -COOH groups
dioyl chlorides can also be used to react with the diamine instead of the acid
a dioyl chloride contains 2 -COCl groups
condensation reaction occurs and forms an amide link
describe the formation of polyamides from monomers
using aminocarboxylic acids, you can carry out a condensation reaction/polymerisation using only one monomer which provides both of the function groups necessary for an amide/peptide link
condensation reaction occurs and forms an amide link/bond with the release of a water molecule
describe the formation of polyamides from aminocarboxylic acids
When a section of polymer is presented, the monomers can be identified by considering the small molecules expelled from the monomers
If a water molecule is expelled, the -OH must have been from an acid group
The hydrogen atom may be from an amine group of a monomer.
If the molecule was hydrochloric acid (HCl), a dioyl chloride monomer may have been used
how can the monomers that make up a condensation polymer be identified
polyalkene chains are non-polar and saturated
this makes them chemically inert, and therefore, non-biodegradable
(Poly)alkenes can be melted and recycled for new uses
However, even in the new applications, the (poly)alkenes are not biodegradable
Recycling plants can burn used plastic materials
The energy released from burning can be used to generate electricity
Burning plastics in oxygen releases carbon dioxide and water (complete combustion) which can contribute to global warming
describe the biodegradability of poly(alkenes)
Polyesters and polyamides are biodegradable polymers for a number of reasons
One such reason is their ability to breakdown with the use of light
Carbonyl groups (C=O) along polymer chains are able to absorb energy from the Electromagnetic Spectrum, in particular ultraviolet (UV) light
Absorbing UV light weakens the carbonyl areas of polymers and breaks them down into smaller molecules
describe the photodegradation of polymers
Despite this ability being a great advantage of polyesters and polyamides, it may pose problems when the polymers are repurposed
When applied to a new use, the biodegradability could give a weaker polymer
Breaking down polymers also poses another challenge
Once used, polymeric materials are taken to landfill sites where many other materials are piled on top of each other
This could mean that photodegradable polyesters or polyamides do not have access to UV light in order to break down naturally
what are the disadvantages of photo degradability
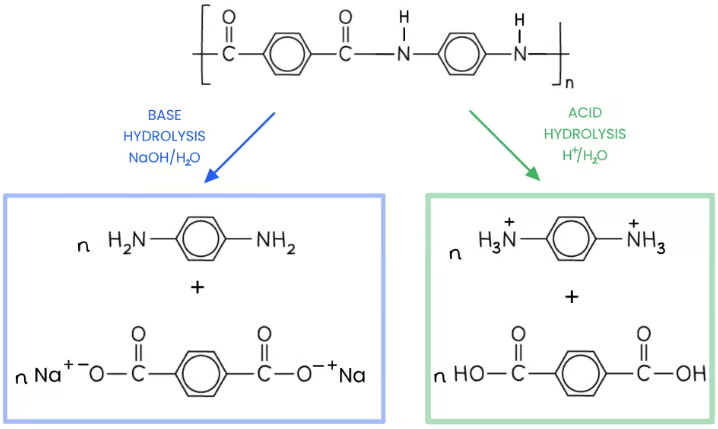
Hydrolysis is the breakdown of molecules using water
In acidic hydrolysis, an acid (such as hydrochloric acid) acts as the catalyst
Polyamides such as Kevlar are heated with dilute acid
This reaction breaks the polyamide into a dicarboxylic acid and ammonium ions
Alkaline hydrolysis
The polyamide is heated with a species containing hydroxide ions (eg. sodium hydroxide)
This breaks the polymer into the sodium salts of its monomers (dicarboxylic acid salt and diamines
describe the hydrolysis of polyamides to biodegrade them
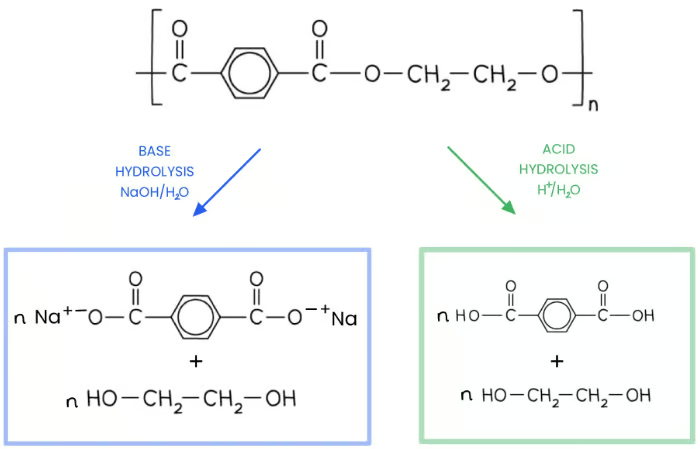
Ester linkages can also be degraded through hydrolysis reactions
Acid hydrolysis forms the diol and dicarboxylic acid that were used to form the polyesters
Alkaline hydrolysis forms the diol and dicarboxylic acid salt
describe the hydrolysis of polyesters to biodegrade them