E3 Radioactive decay
1/24
Earn XP
Description and Tags
Radioactive
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
E3.1 Isotopes
Element = fixed nr of protons in atom
Diff nr of neutrons → isotopes
Know: hydrogen, deuterium, titrium (0,1,2 neutrons)
Z is constant, A is not.
Imbalance of prot/neutr → unstable → decay and emit radiation → stable
E3.2 Relative atomic mass
Average mass of element based on presence/abundance of different isotopes of element in a substance.
E3.3 Percentage abundance of isotope
How many of 100 atoms of this element are of this certain isotope → used to calc. relative atomic mass.
E3.4 Carbon Dating
Ratio of stable carbon-12 atoms to unstable carbon-14. Compare dead to living → see how many 14→12 (we know the rate so find the t)
Use half lifes
1n + 147Nitrogen → 146Carbon + 1p
E3.5 Radioactive decay
Spontaneous breaking of a nucleus to form more stable nucleus, resulting in emission of α, β or γ particle. Its random. can happen at any moment.
Spontaneous: not influenced by env. factors, random: time non-predictable
with lot of nuclei, possible to statistically predict using probs. for group
E3.6 Background Radiation
Ionising radiation present in environment, by natural/artificial sourceE
E3.7 Decay types
Imbalance of protons, neutrons, or energy → emit particles/radiation
alpha α
beta β
gamma γ
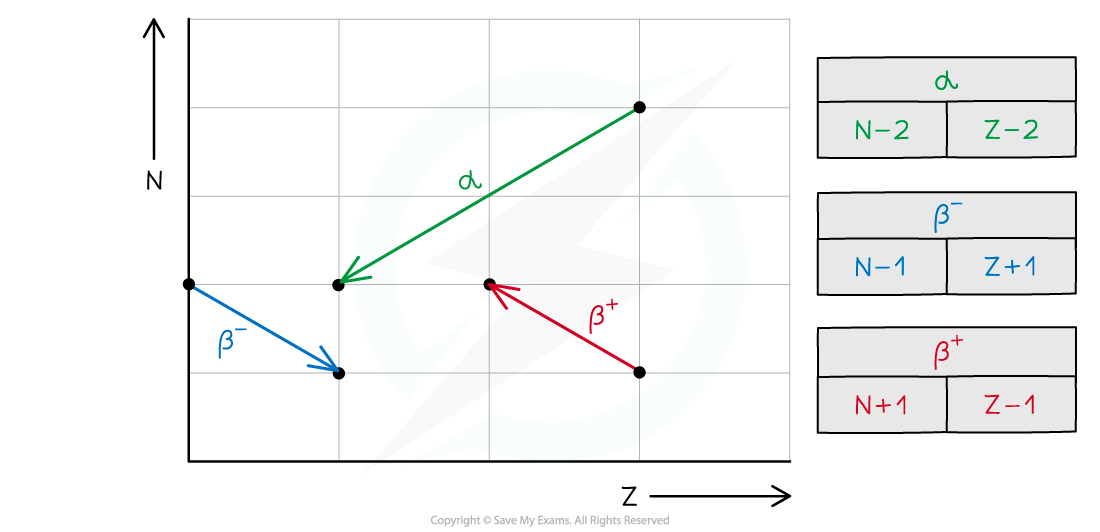
E3.8 Alpha decay
α : high energy He nucleus → 2prot, 2neutr → 4u mass, +2e charge
42α
emitted by large, unstable nuclei with too many nucleons (prot&neut)
Parent nucleus(AZX)→ 42α + Daughter nucleus (A-4Z-2Y)
AZX→ 42α + A-4Z-2Y
E3.9 Beta-minus decay
β- : high energy e-, mass of 0.0005u and charge -1e
0-1β-
Too many neutrons: neutron turns into proton and emits electron + anti-neutrino → proton nr+1, nucleon nr constant
n → 0-1β- + v + p
AzX → 0-1β- + v + AZ+1Y
the v is an antineutrino, we do later…
E3.10 Beta-plus decay
β+ : high energy positron: antimatter of electron: 0.0005u, +1e charge
0+1β+
Too many protons: proton turns into neutron and emits positron + neutrino → proton nr-1, nucleon nr constant
p → 0+1β+ + v + n
AzX → 0+1β+ + v + AZ-1Y
E3.11 Gamma radiation
γ = high energy EM radiation = photon
Emitted by nuclei that need to lose some energy
00γ
proton and nucleon nr both constant.
AZX → 00γ + AZY
E3.12 Ionising ability of α, β, γ
Measure of # of ionisation: when nuclear radiation pass through material
If radiation collides w. atom → it might knock out e- : ionising atom.
Highly dangerous for living cells, α>β>γ
α: 3-5 cm traveled, highly ionising, weakly pen. , pass through paper
β: 20cm-3m, moderately, mod. penet, pass through alu foil
γ: infinite…, weakly ionizing, highly penetrating, pass through thick lead
E3.13 Penetrating power
distance nuclear radiation will travel before losing all its energy .
Shorter → shorter range in air. Highly ionising → pen. power
E3.14 Deflection in E and B-fields
In E field, β+ and α → - plate, β- to + plate. γ non deflected
In B-field, also.
E3.15 Radioactive decay equations
too many n’s : β- : AzX → 0-1β- + v + AZ+1Y
too many p’s: β+ : AzX → 0+1β+ + v + AZ-1Y
too many p/n’s: α. : AZX→ 42α + A-4Z-2Y
too much E: γ; AZX → 00γ + AZY
E3.16 (anti-) neutrinos
Subatomic particles, no charge, no mass, also emitted…
antineutrino in β- decay, neutrino in β+ decay. no relevance,except conserv E
Evidence: α have discrete E, β continuous, shared between β and v

E3.17 Activity + half life explained
Activity: nr of nuclei decaying in given time: Bq: 1 decay/s
Rate at which activity decreases is predictable:
Half life- time taken for half of nuclei to decay = half the activity aswell, constant, so t to go from 500→250 = t from 250 → 125.
Each isotope of each element has unique halflife, from 0.00001 s to 100000 years.
E3.18 Decay constant
λ : prob that individual nucleus decays per second; use average decay rate
Activity A = ΔN/Δt = -λN (N=nr of undecayed nuclei in sample, ΔN = decayed)
Greater λ → greater A. - bc nr of nuclei remaining decreases over time.
N = Noe-λt
A = Aoe-λt
C = Coe-λt (count rate…)
t½ = ln 2/λ
draw logN vs t½ give linear graph with formula: ln N = -λt + ln No
Exponential decay: steeper slope: larger λ, N vs t, start at No
E3.19 Mass defect
Diff between measured mass of nucleus vs. sum of masses of constituents
Δm = Z(mp) + (A-Z)(mn) - mtot measur.
We find that Δm = positive.
E3.20 Mass energy equivalence & binding energy
All masses have energy. Constituents of nucleus have larger m/E than final. Difference in energy: ΔE = Δmc2, where m is the mass defect.
Binding energy: energy required to break nucleus into constituent p + n; making nuclei from system of pure p+n, releases E.
Can do binding energy per nucleon, or total.
ΔE = mc2 is used in fission, fussion, weapons and particle collisions.
Careful with MeV, J, u etc…
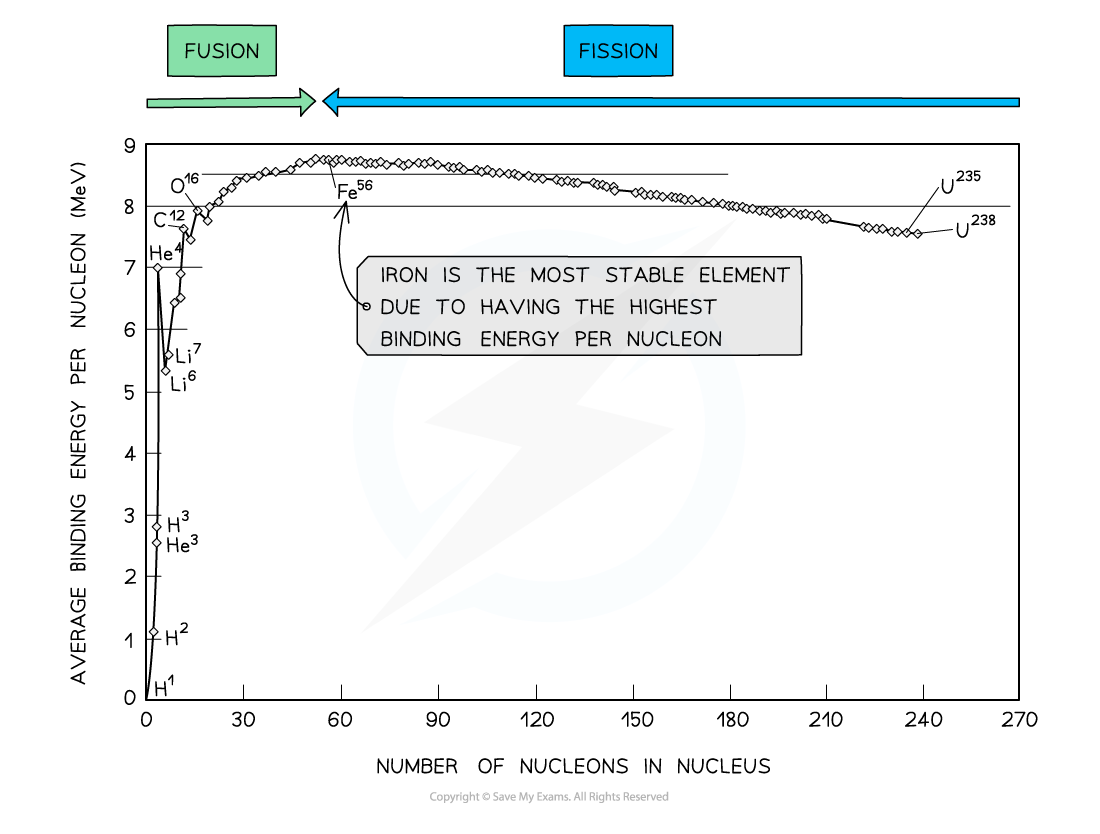
E3.21 Binding energy per nucleon curve
More E/nucleon → more stable → more E to sep nucleons in this nucleus
first fusion: A+B→C, last: C→A+B. Iron most stable. : both release E
Greater mass defect → stabler.
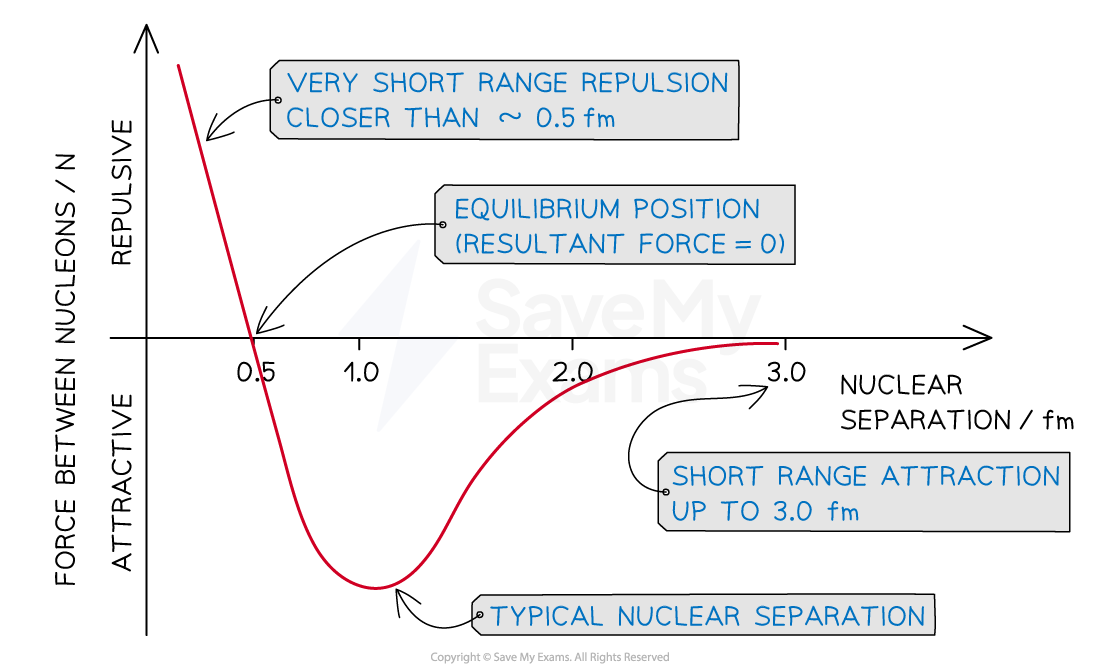
E3.22 Strong nuclear force
Repulsive electric between + charges
Attractive grav bc of mass (neglible)
Strong nuclear force:attractive & stronger than electric repulsive
Acts between quarks (what prot/neut are made up of)
Strength depends on seperation between nucleons. Repulsive when too close, very attractive when mid, then slightly attractive. Very small range.
E3.23 Nuclear stability
Band of nuclear stabillity graph. First more stable when N=Z (up to z=20), then more neutrons than protons. Under line of stability;β+ decay, over: β-. Top has mostly α.

E3.24 Nuclear Energy levels
Nucleus can exist in excited state similar to e-. Once unstable nucleus decays: can emit gamma photons. Allowing the nucleus to lose energy. This happens after daughter nucleus is in excited state after decaying. Short excitation and moves to groun state quickly, via 1 or more steps.
Get weird graphs ???