Bond Angles
0.0(0)
Card Sorting
1/11
Earn XP
Description and Tags
Hybridization + shape name + bond angle without degrees
Last updated 1:44 PM on 3/4/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
1
New cards
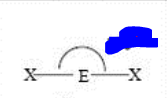
bp: 2 lp: 0
sp linear 180
2
New cards
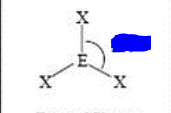
bp: 3 lp: 0
sp2 trigonal planar 120
3
New cards
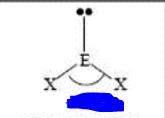
bp: 2 lp: 1
sp2 bent <120
4
New cards
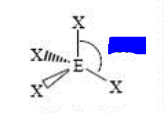
bp: 4 lp: 0
sp3 tetrahedral 109
5
New cards
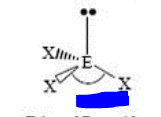
bp: 3 lp: 1
sp3 trigonal pyramidal <109
6
New cards
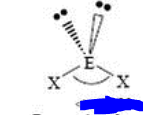
bp: 2 lp: 2
sp3 bent <109
7
New cards
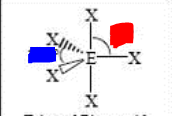
bp: 5 lp: 0
sp3d trigonal bipyramidal 120 and 90
8
New cards
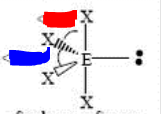
bp: 4 lp: 1
sp3d seesaw <120 and <90
9
New cards
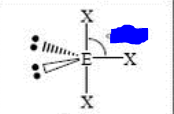
bp: 3 lp: 2
sp3d T-shape 90
10
New cards
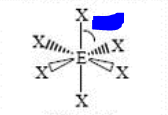
bp: 6 lp: 0
sp3d2 octahedral 90
11
New cards
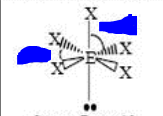
bp: 5 lp: 1
sp3d2 square pyramidal <90
12
New cards
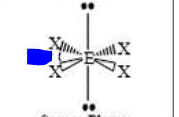
bp: 4 lp: 2
sp3d2 square planar 90