physical chemistry
1/140
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
141 Terms
what aspects must you mention with ionisation energy trends?
nuclear charge, atomic radius, electron shielding
rate equation (with k)
rate = k[A]^m[B]^n
1st order
changing the concentration changes the rate by the same
0 order
if changing the concentration has no effect on the rate
2nd order
if changing the concentration has a squared effect on the rate. e.g doubling concentration quadruples rate
what model of the atom do trends in ionisation energy support?
the bohr model supports these trends
giant covalent lattice properties explained
high melting and boiling points and insoluble due to strong covalent bonds, can only conduct if there are delocalised electrons like graphene and graphite
trend in reacivity down group 2
more reactive as you go down due to a decrease in ionisation energy (more electron shielding, increased atomic radius)
trend in alkalinity of group 2 hydroxide solutions
alkalinity increases as solubility increases down the group
group 2 compound uses
Ca(OH)2 to neutralise soils in agriculture, Mg(OH)2 and CaCO3 as antacids in treating indigestion
halogen boiling point trend
boiling point increased and reactivity decreases down the group due to increased electrons so stronger london forces
halogen reactivity in terms of first ionisation
harder to form -1 ions down the group due to lower nuclear attraction, higher atomic radius, and more elctron shielding so it’s harder to gain another electron.
what is a disproportionation reaction?
the same element is both oxidised and reduced in the same reaction
benefits and risks of chlorine in water treatment
kills harmful bacteria, toxic gas, chlorinated hydrocarbons are carcinogenic
silver nitrate and Cl- ions appearance
white precipitate
silver chloride and weak ammonia
dissolves precipitate. clear solution
silver nitrate and Br- ions reaction appearance
cream precipitate
silver nitrate and I- ions appearance
yellow precipitate
silver bromide reaction with ammonia
no reaction with weak, dissolves to clear solution with strong
silver iodide with ammonia
no reaction no matter strong or weak
disproportionation examples
Cl2 in water makes HClO +1 and HCl -1, Cl2 and cold dilute NaOH NaCl -1 and NaClO +1
ammonia NH3 test
mix with warm dilute NaOH(aq) if positive damp red litmus paper turns blue
is bond breaking exo or endothermic
endothermic
is bond making exo or endothermic
bond making is exothermic
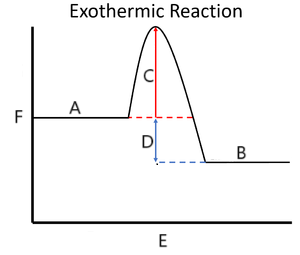
label the diagram
A reactants
B products
C Ea activation energy
D ΔH enthalpy change
E reaction progress
F enthalpy kJ/mol
Exothermic
enthalpy change ΔH
the change in heat energy of a substance expressed at a constant pressure
how to calculate enthalpy change
products - reactants
standard enthalpy of combustion ΔHc
the enthalpy change when one mole of a compound is burned completely in oxygenated standard conditions
what is ΔHθ
the change in heat energy under standard conditions 100kPa 298K
amount of heat gained or lost by a sample
q=mc∆T
q is heat lost/gained (J)
m is the mass of water/solution (g)
c specific heat capacity of water (J/g/K)
∆T change in temp (K)
enthalpy change of reaction ΔHºr
the enthalpy change of a reaction in moles with reactant and products in standard states and conditions
average bond enthalpy
the average enthalpy change that takes place when breaking by homolytic fission one mole of a given type of bond in the molecules of a gaseous species
enthalpy change of formationΔHºf
the enthalpy change when one mole of a compound is formed from its elements in their standard states under standard conditions
standard enthalpy of formation of elements in their natural states
0kJ/mol
standard enthalpy change of neutralisation ΔHºc
the enthalpy change that accompanies the reaction of an acid by a base to from one mole of H2O(l) with all reactants and products in their standard states under standard conditions
standard conditions
298k/25c 1atm pressure/101kPa 1moldm³
Hess’s law
if a reaction can take place by 2 routes, the total enthalpy change is the same for each route, providing that the start and end conditions are the same
endothermic reaction enthalpy change
positive
exothermic reaction enthalpy change
negative
what is the collision theory
for a chemical reaction to take place the particles need to collide with each other in the correct orientation and enough energy
ineffective collision
particles collide in the wrong orientation or when they don’t haver enough energy and bounce off each other without causing a chemical reaction
what is collision frequency
number of collisions per unit time
effect of concentration on reaction rate
increase in collision frequency and rate of reaction
effect of pressure on reactions
increase
reaction rate formula
change in amount of reactants or products (mol dm^-3) / time (s)
role of a catalyst
a substance that increases the rate of reaction by providing the particles with an alternative mechanism with a lower activation energy
types of catalyst
homogeneous and heterogeneous
homogeneous catalyst
the catalyst is in the same phase as the reactants (e.g everything is in solution)
heterogeneous catalyst
the catalyst is in a different phase to the reactants (e.g reactants are gases catalyst is a solid)
catalyst benefits
lower temparature and pressure can be used
reduced energy demand
fewer CO2 emissions from fossil fuels
use reactions with better atom economy '
less undesired products and hazardous solvents and reactants
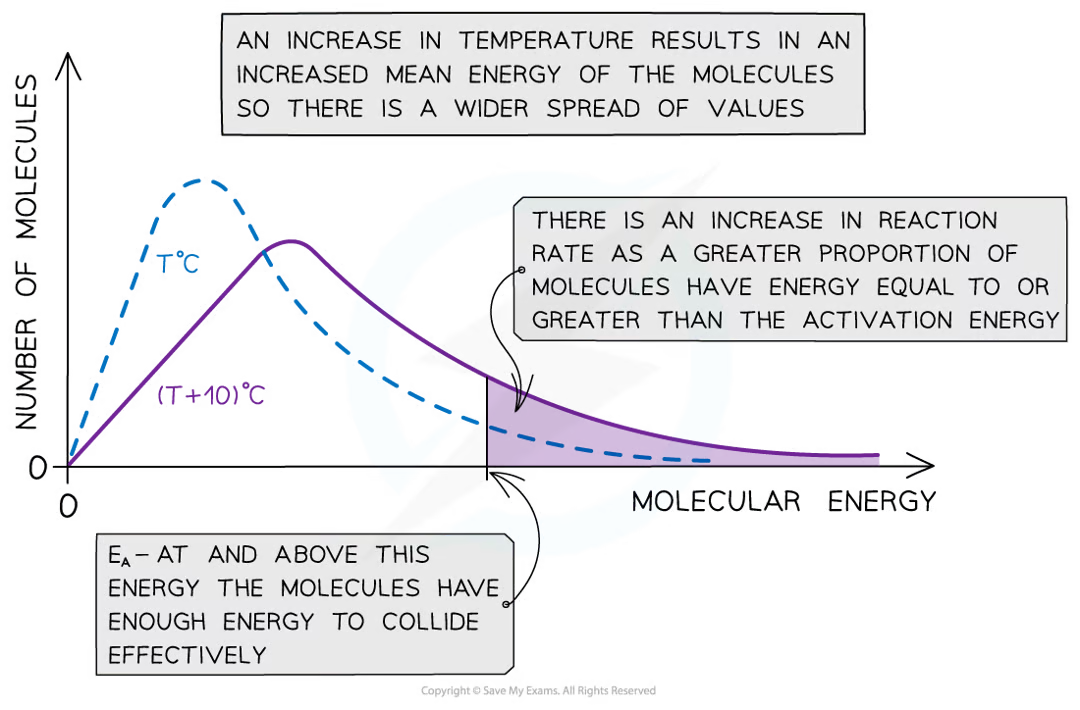
what is a maxwell distribution curve
a graph that shows the distribution of energies at a certain temparature
in a gas some particles will have very low energy, some very high, most in between
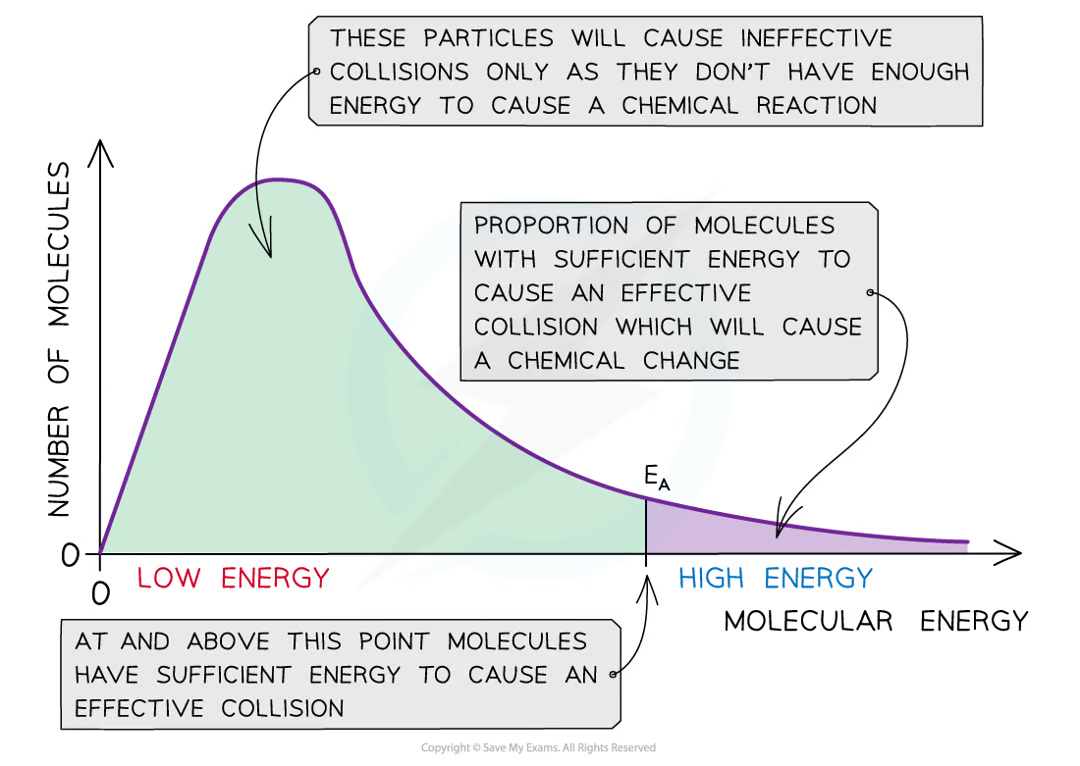
what happens to the Boltzmann distrinution curve in increasing temparatures
it flattens and the peak shifts to the right
dynamic equilibrium
the rate of the forward reaction is equal to the rate of the backward reaction in a closed system and the concentrations of each are constant
closed system
none of the reactants or products escape form the reaction mixture
open system
matter and energy can be lose to the surroundings
equilibrium position
relative amounts of products and reactants in an equilibrium mixture (to left is more reactants right more products)
Le Chatelier’s principle
if a change is made to a system in dynamic equilibrium the position of the equilibrium moves to counteract this change
equilibrium shift in concentration of reactant change
increse to right, decrease to left
equilibrium shift in pressure changes
increase shifts to direction with less gas molecules to decrease the pressure, decrease vice versa
effect of temparature on equilibrium
increase moves to endothermic side, decrease moves to exothermic
catalyst in dynamic equilibrium
increase the rate of the forward and backward reaction equally. only cause a reaction to reach equilibrium faster. no effect on position of equilibrium
the haber process
synthesis of ammonia with the equation
N2(g) + 3H2(g) ⇌ 2NH3(g)
maximising ammonia yield in the haber process
increase in pressure because there is less gas molecules in the forward but high pressure expensive so 200atm chosen
lowered temparature because exothermic reaction but too low not enough activation energy so compromise of 400-450c chosen
iron catalyst
the contact process
the synthesis of sulfuric acid with equation
S(s) + O2(g) → SO2(g)
2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
SO3(g) + H2SO4 → H2S2O7 + H2O + 2H2SO4
maximising sulfuric acid yield in the contact process
increase pressure because less gas molecules but only carried out at 1atm because higher is unnecessary and expensive
lowered temperature because reaction is exothermic but compromise of 450c is used
vanadium(V) oxide catalyst
the equilibrium constant expression
an expression that links the equilibrium constant, K to the concentrations of reactants and products at equilibrium taking the stoichiometry of the equation into account
how to write equilibrium constant expression
Solids are ignored in equilibrium constant expressions
The equilibrium constant, K, of a reaction is specific to a given equation
The Kc of a reaction is specific and only changes if the temperature of the reaction changes
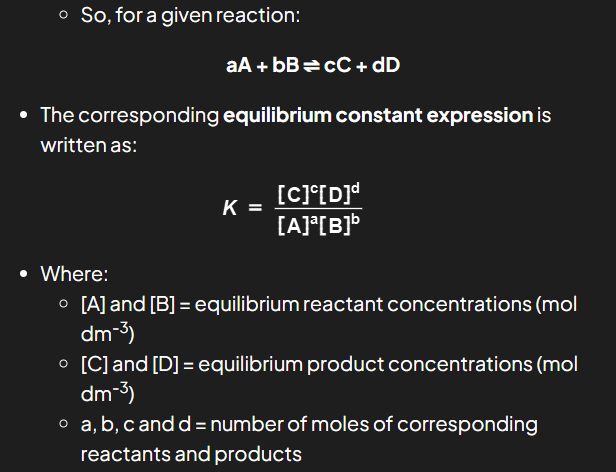
using Kc for equilibrium position
The magnitude of Kc indicates the relative concentrations of reactants and products in the mixture
If Kc is very large (Kc >>1) the equilibrium lies to the RHS so the reaction mixture contains mostly products
If Kc is very small (Kc << 1) the equilibrium lies to the LHS so the reaction mixture contains mostly reactants
If Kc is close to 1 the mixture contains a similar concentration of both reactant and products
how is the periodic table arranged
-by increasing atomic number
-in periods showing repeating trends in physical and chemical properties (periodicity)
-in groups having similar chemical properties
s block elements
group one and two (because their electron configuration ends in s)
p block elements
groups 3-8 (because their electorn configuration ends in p)
d block elements
transition metals
first ionisation energy
the removal of 1 mol of electrons from 1 mol of gaseous atoms
lattice enthalpy definition ∆LEH
enthalpy change from the formation of 1 mol of an ionic lattice from gaseous ions
how can latttice enthalpy tell use the strength of bonds
higher enthalpy stronger bonds
enthalpy change of formation
the enthalpy change that occurs when 1 mole of the substance is formed from its elements in their standard states
enthalpy change of atomisation
ΔatH is the enthalpy change when one mole of gaseous atoms is formed from the element in its standard state, under standard conditions.
electron affinity
enthalpy changes when one mole of electrons is added to one mole of gaseous atoms/ions
enthalpy of solution definition
the enthalpy changfe when 1 mol of a solute dissolves ∆solH
enthalpy change of hydration
the enthalpy change when 1 mol of gaseous ions dissolves in water ∆hydH
factors affecting exothermic value of lattice enthalpy
-ionic charge - higher more attraction
-ionic radius - smaller more attraction
what is entropy
a measure of the dispersal of energy in a system which is greater the more disordered a system
when is a reaction thermodynamically stable
-absolute 0 (0K, maximum order)
-gibbs free energy is positive
change in entropy when melting
-increase - smaller than liquid to gas
change in entropy when boiling
-increase - larger than from solid to liquid
change in entropy when condensing or freezing
decrease
change in entropy when moles of gas increase
increase
change in entropy when number of gas moles decrease
decrease
Gibbs free energy equation
∆G = ∆H – T∆S
feasibility of positive ∆H negative ∆S
∆G always positive. never feasible
feasibility of negative ∆H positive ∆S
∆G always negative. reaction feasible
feasability of negative ∆H negative ∆S
∆G negative at low temp. feasible at low T
feasibility at positive ∆H positive ∆S
∆G negative at high temp, feasible at high T
what range must ∆G be for a reaction to be feasible
0 or less
limitations of predictions made for feasability
-decomposition of hydrogen peroxide gibbs is -117kJmol-1
-doesn’t decompose spontaneously. very high activation energy so reacts very slowly
carbonate test
-in a test tube add dilute nitric acid to the sample to be tested
-if you see bubble, could be carbonate
-bubble gas through limewater, Ca(OH)2
-will turn limewater cloudy if positive
sulfate test
-add aqueous barium chloride or barium nitrate
-Ba2(aq) + SO42-(aq) → BaSO4(s)
-forms white precipitate which works because it is highly insoluble
halide test
-add silver nitrate solution
-precipitate formed if positive - white if Cl, cream if Br, yellow if I
-can be difficult to tell apart, so further step
-weak ammonia solution dissolves AgCl, strong dissolves AgBr, AgI not affected
correct order for anion test
-carbonate
-sulfate
-halide
test for ammonia ion NH4+
-add aqeuous sodium hudroxide NaOH to solution for testing
-ammonia gas is produced
-mixture is warmed, gas is released
-use pH indicator paper, will turn damp paper blue as its alkalaine