Unit 3 AP BIO Exam Review
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
84 Terms
TOPIC 3.1 Enzyme Structure
ENDURING UNDERSTANDING: The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.D Describe the properties of enzymes.
ESSENTIAL KNOWLEDGE
ENE-1.D.1 The structure of enzymes includes the active site that specifically interacts with substrate molecules.
ENE-1.D.2 For an enzyme-mediated chemical reaction to occur, the shape and charge of the substrate must be compatible with the active site of the enzyme.
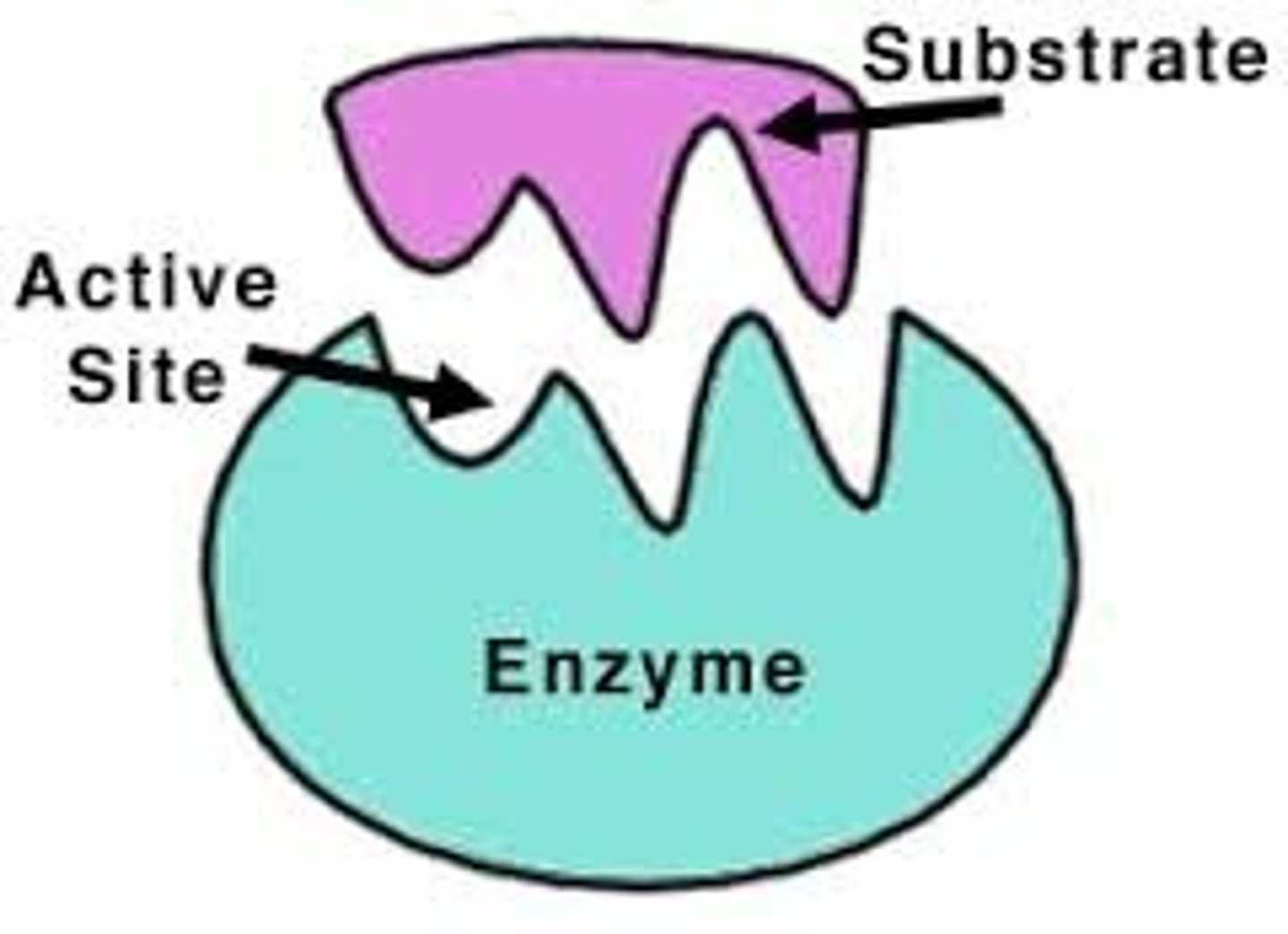
This is an enzyme →
Label the active site.
Draw a substrate molecule that would bind to this enzyme.
Draw a substrate molecule that would NOT bind to this enzyme.
- any substrate that is not the exact shape of the active site
- needs to fit like a lock and key
What type of biomolecule are enzymes? ________________ What is the monomer? ______________
enzyme = proteins
monomer = amino acids
The most important thing about an enzyme its S__________! An enzyme must have the right S__________ in order to do its job!
- shape
- shape
This enzyme has an amino acid at the center of the active site with a positively charged R group.
Predict - how would the function of this enzyme be affected if a mutation led to a negatively charged amino acid being in that location? Explain your thoughts.
Predict - how would the function of this enzyme be affected if a mutation led to a different, but also positively charged amino acid being in that location? Explain your thoughts.
a negatively charged amino acid (R group) would likely prevent the enzyme from functioning, The positive charge would probably disrupt the shape of the enzymes active site, preventing binding of the substrate.
it is possible that the substitution of a different but also positively charged R groups, would not impact the shape or function of the enzyme. The charge has not changed, so the protein is likely to fold properly, and interact with the substrate in the same way
TOPIC 3.2 Enzyme Catalysis
ENDURING UNDERSTANDING: The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.E Explain how enzymes affect the rate of biological reactions.
ESSENTIAL KNOWLEDGE
ENE-1.E.1 The structure and function of enzymes contribute to the regulation of biological processes—
Enzymes are biological catalysts that facilitate chemical reactions in cells by lowering the activation energy.
In your own words, what is the 'activation energy' of a reaction?
the amount of energy required to make a chemical reaction happen.
Below is an 'enzymatic reaction' - taking one plastic straw and breaking it into two pieces of straw (AB → A + B). The enzyme doing this reaction is the scissors.
The reaction rate speed would lower because without the enzyme (scissors) it would take a lot of energy to break down the straw in half, therefore take a long time to break each one- slowing down the reaction rate( number of straw per second) with the enzyme reaction rate is faster
Label the Biological Catalyst, the Substrate and the Product of this reaction. Label the 'active site' on the enzyme.
AB ----> A + B
substrate products
Given a box of straws and a pair of scissors you would be able to cut the straws (produce the p______________ of the reaction) very quickly.
product
Predict - What would happen to the reaction rate if you did NOT have the scissors, and you were breaking the straws by hand? The reaction rate (speed) would _______________ Why? Explain in terms of the 'activation energy' of the reaction.
slow because it takes more energy to break the straw
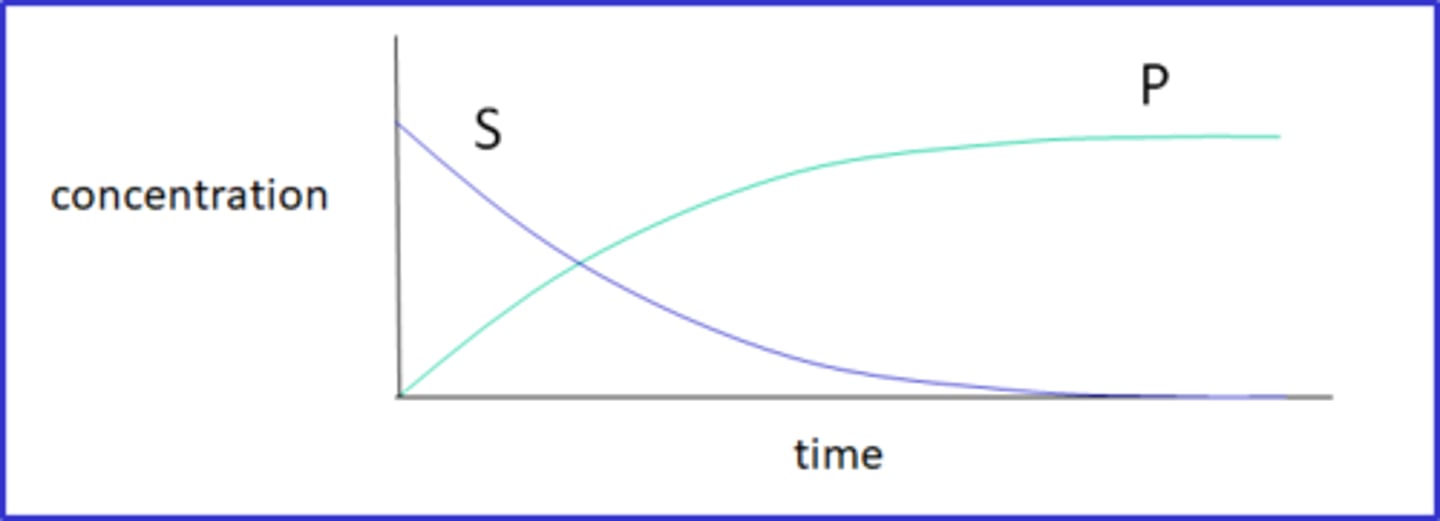
Label the Substrate and Product of one graph.
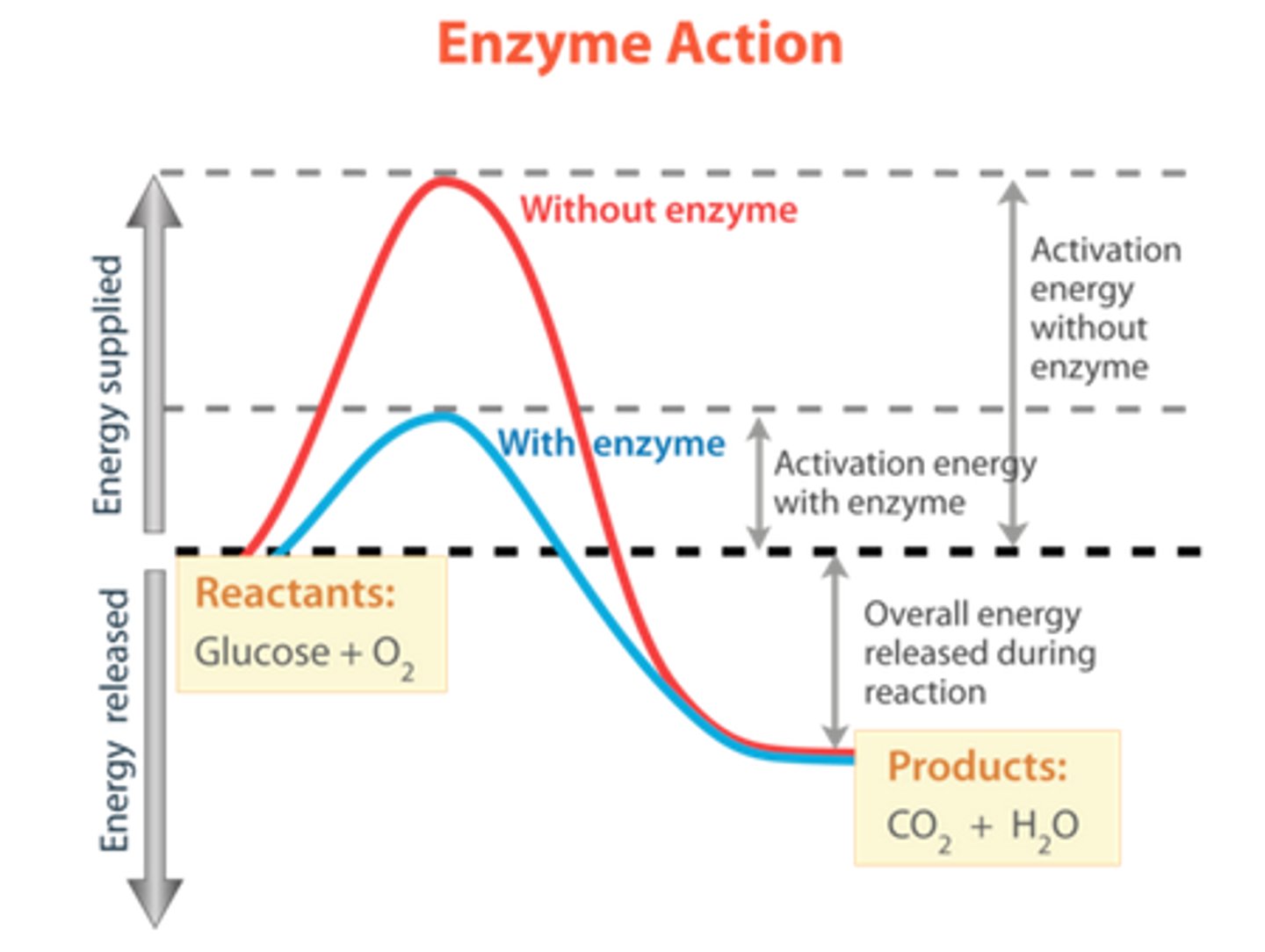
Which graph on the right shows a reaction WITH an enzyme? Which shows a reaction WITHOUT an enzyme? Label them.
TOPIC 3.3 Environmental Impacts on Enzyme Function
ENDURING UNDERSTANDING: The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.F Explain how changes to the structure of an enzyme may affect its function.
ESSENTIAL KNOWLEDGE
ENE-1.F.1 Change to the molecular structure of a component in an enzymatic system may result in a change of the function or efficiency of the system—
a. Denaturation of an enzyme occurs when the protein structure is disrupted, eliminating the ability to catalyze reactions.
b. Environmental temperatures and pH outside the optimal range for a given enzyme will cause changes to its structure, altering the efficiency with which it catalyzes reactions.
ENE-1.F.2 In some cases, enzyme denaturation is reversible, allowing the enzyme to regain activity
LEARNING OBJECTIVE
ENE-1.G Explain how the cellular environment affects enzyme activity.
ESSENTIAL KNOWLEDGE
ENE-1.G.1 Environmental pH can alter the efficiency of enzyme activity, including through disruption of hydrogen bonds that provide enzyme structure.
**See the equation for pH on your formula sheet
X Students must understand the underlying concepts and applications of the pH equation, but performing calculations using this equation are beyond the scope of the AP Exam.
ENE-1.G.2 The relative concentrations of substrates and products determine how efficiently an enzymatic reaction proceeds.
ENE-1.G.3 Higher environmental temperatures increase the speed of movement of molecules in a solution, increasing the frequency of collisions between enzymes and substrates and therefore increasing the rate of reaction.
ENE-1G.4 Competitive inhibitor molecules can bind reversibly or irreversibly to the active site of the enzyme. Noncompetitive inhibitors can bind allosteric sites, changing the activity of the enzyme.
Functional Enzyme
Draw an enzyme that looks like Pac Man.
Now draw a substrate molecule that would fit into the enzyme's active site (mouth).
Denatured Enzyme
Draw the Pac Man enzyme again, but demonstrate that it has been denatured.
Draw the same substrate
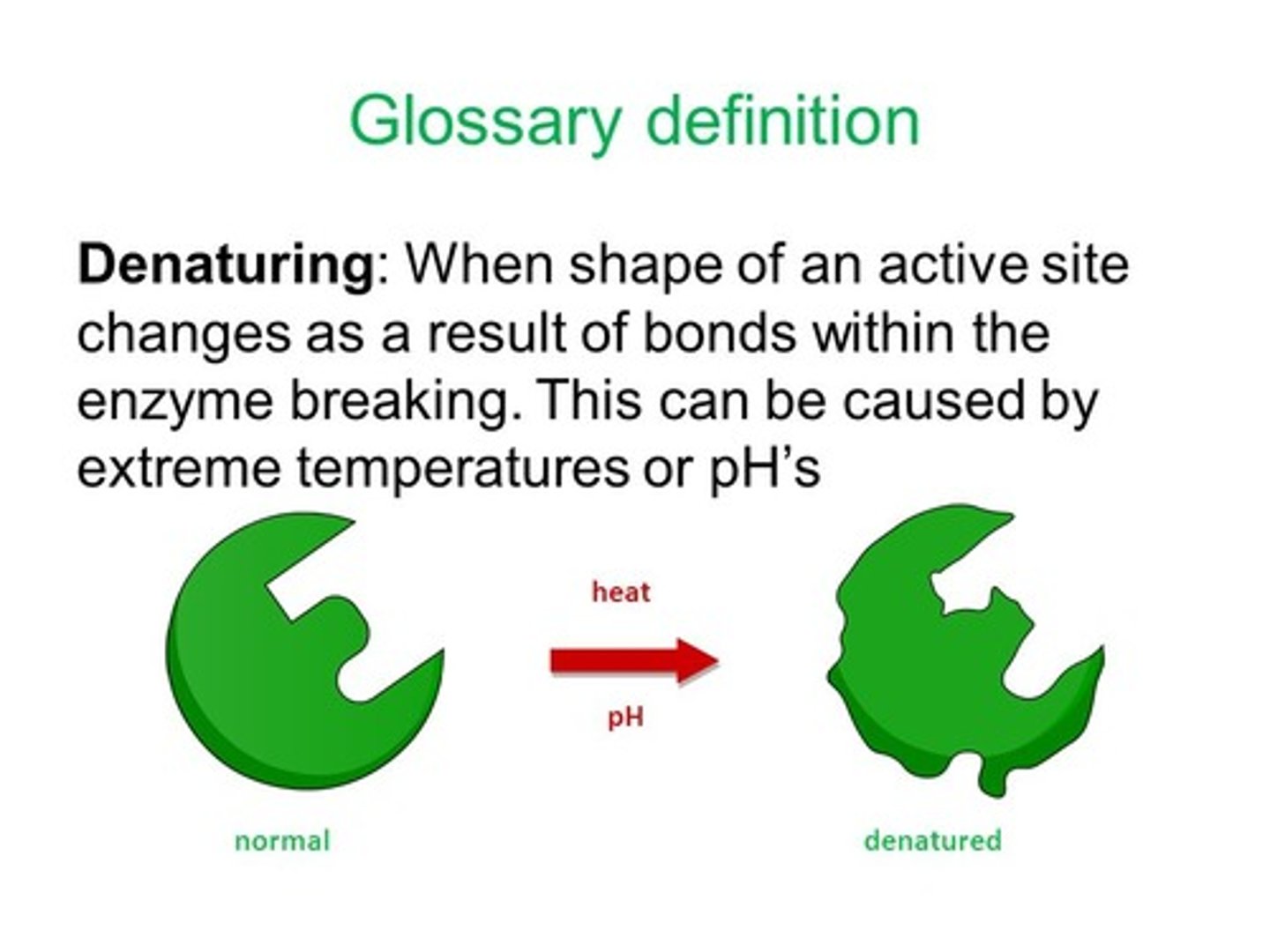
How does denaturation impact the enzyme's ability to catalyze this reaction? Explain using the diagram above.
For enzymes a change in structure means a change in the active site. If the active site changes shape the substrate is no longer able to bind to it. this means no catalyzing of the reaction will take place.
How is the reaction rate of an enzyme affected by temperature? By pH? Draw graphs below of the reaction rate in different temperatures and pH.
-at low temperature minimal enzyme activity-increasing temperature will increase speed, increasing enzyme activity-Higher temperatures will cause enzyme stability to decrease, resulting in denaturation
-changing the pH will alter the charge of the enzyme, changing the solubility and shape-changing the shape of the active site will diminish its ability to bind to the substrate-enzymes have an optimum pH and moving outside the range will always result in a diminished rate of reaction
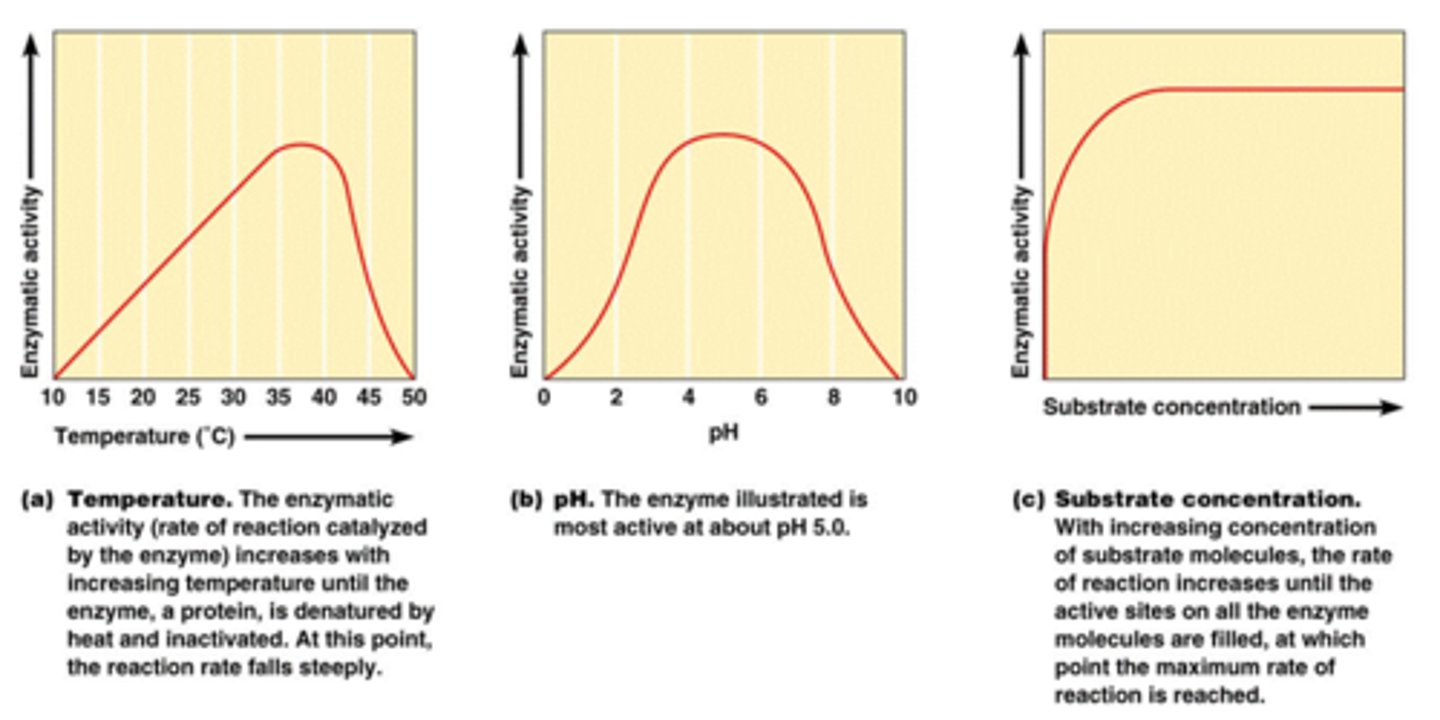
A pH of 2 is ______ times as acidic as a pH of 3.
A pH of 2 is ______ times more acidic than a pH of 4.
10x stronger at 2 then 3
100x stronger at 2 then 4
Why/how do acids and bases (outside of optimum) denature enzymes?
cause amino acids' component atoms and molecules to ionize. This can make an enzyme change shape. These shapes determine function, so changing the shape can impair the enzyme's function, preventing it from speeding up chemical reactions.
Here is the formula for an enzymatic reaction of catalase:
H2O2 → H2O + O2
What can we directly measure that will tell us the reaction rate of catalase? (there are three!)
-how much water is produced
-how much oxygen is produced
-how much hydrogen peroxide is used
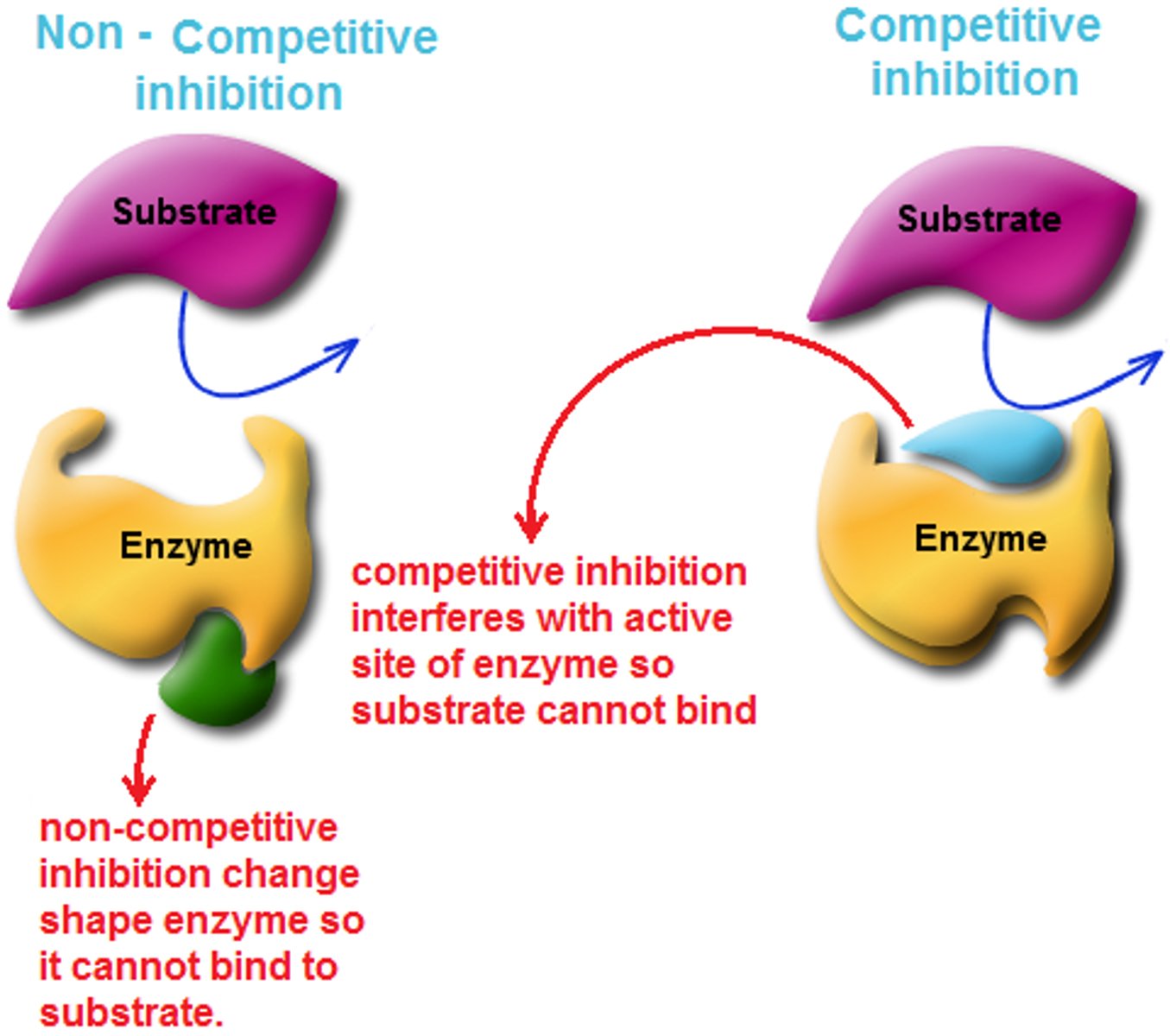
Some enzymes can become inactive when particular inhibitors bind to them. Inhibitors prevent the substrate from.....
binding to the active site on the enzyme
Competitive inhibitor vs Noncompetitive (allosteric) inhibitor.
Draw an enzyme bound to each type of inhibitor. Make it simple, like the 'pac man' enzyme. Draw the substrate in both cases as well, demonstrating that it can no longer bind to the active site
Competitive Inhibitor: an inhibitor that resembles the normal substrate binds to the enzyme, usually at the active site, and prevents the substrate from binding (takes its place)
Noncompetitive Inhibitor (Allosteric): an inhibitor that binds to an allosteric site separate from the active site of substrate binding. Thus in noncompetitive inhibition, the inhibitor can bind its target enzyme regardless of the presence of a bound substrate. (changes shape of active site but doesn't take the place)
TOPIC 3.4 Cellular Energy
ENDURING UNDERSTANDING: The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.H Describe the role of energy in living organisms.
ESSENTIAL KNOWLEDGE
ENE-1.H.1 All living systems require constant input of energy.
ENE-1.H.2 Life requires a highly ordered system and does not violate the second law of thermodynamics—
a. Energy input must exceed energy loss to maintain order and to power cellular processes.
b. Cellular processes that release energy may be coupled with cellular processes that require energy.
c. Loss of order or energy flow results in death.
X Students will need to understand the concept of energy, but the Gibbs free energy equation is beyond the scope of the coure and the AP Exam.
ENE-1.H.3 Energy-related pathways in biological systems are sequential to allow for a more controlled and efficient transfer of energy. A product of a reaction in a metabolic pathway is generally the reactant for the subsequent step in the pathway.
Explain why free energy is necessary to living things. Include the word 'entropy' and 'homeostasis' in your answer.
Free energy increases entropy. Homeostasis ensures that too much entropy doesn't kill the organism.
entropy: is a measure of randomness or disorder in a system. (2nd law of thermodynamics)
What happens when organisms get less free energy than is required to stay alive?
organisms may not be able to utilize functions required for life, and may die
What is the first law of thermodynamics?
(Energy can be transferred and transformed, but it cannot be created or destroyed.)
states that heat is a form of energy, and thermodynamic processes are therefore subject to the principle of conservation of energy. This means that heat energy cannot be created or destroyed. It can, however, be transferred from one location to another and converted to and from other forms of energy.
What is the second law of thermodynamics?
The principle stating that every energy transfer or transformation increases the entropy of the universe.
states that as energy is transferred or transformed, more and more of it is wasted. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state.
Name one cellular process that releases energy: _________________________
This release of energy can be coupled with cellular processes that require energy, like ______________________
cellular resperation:
Glyscolsis = takes energy to start
citric acid cycle = makes more energy
Earth gets a constant supply of free energy from the _________
sun
What do plants (autotrophs) do with that free energy? _________________
photosynthsis: capture light energy from the sun and absorb carbon dioxide and water from their environment. Using the light energy, they combine the reactants to produce glucose and oxygen, which is a waste product. They store the glucose, usually as starch, and they release the oxygen into the atmosphere.
How do we (heterotrophs) get the free energy we need?
eat the plants and other animals
TOPIC 3.5 Photosynthesis
ENDURING UNDERSTANDING: The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.I Describe the photosynthetic processes that allow organisms to capture and store energy.
ESSENTIAL KNOWLEDGE
ENE-1.I.1 Organisms capture and store energy for use in biological processes—
a. Photosynthesis captures energy from the sun and produces sugars.
Photosynthesis first evolved in prokaryotic organisms
Scientific evidence supports the claim that prokaryotic (cyanobacterial) photosynthesis was responsible for the production of an oxygenated atmosphere
Prokaryotic photosynthetic pathways were the foundation of eukaryotic photosynthesis
ENE-1.I.2 The light-dependent reactions of photosynthesis in eukaryotes involve a series of coordinated reaction pathways that capture energy present in light to yield ATP and NADPH, which power the production of organic molecules.
LEARNING OBJECTIVE
ENE-1.J Explain how cells capture energy from light and transfer it to biological molecules for storage and use.
ESSENTIAL KNOWLEDGE
ENE-1.J.1 During photosynthesis, chlorophylls absorb energy from light, boosting electrons to a higher energy level in photosystems I and II.
ENE-1.J.2 Photosystems I and II are embedded in the internal membranes of chloroplasts and are connected by the transfer of higher energy electrons through an electron transport chain (ETC).
ENE-1.J.3 When electrons are transferred between molecules in a sequence of reactions as they pass through the ETC, an electrochemical gradient of protons (hydrogen ions) is established across the internal membrane.
ENE-1.J.4 The formation of the proton gradient is linked to the synthesis of ATP from ADP and inorganic phosphate via ATP synthase.
ENE-1.J.5 The energy captured in the light reactions and transferred to ATP and NADPH powers the production of carbohydrates from carbon dioxide in the Calvin cycle, which occurs in the stroma of the chloroplast.
X Memorization of the steps of the Calvin cycle, the structure of the molecules, and the names of enzymes (with the exception of ATP Synthase) are beyond the scope of the AP Exam
How did cyanobacteria acquire energy? _________________ How did they impact the early Earth?
- photosynthetic. They convert sunlight into energy and produce oxygen as a waste product
- Back then, the Earth's atmosphere didn't have free oxygen in it as it does today. the plants created that through releasing it as waste
What do banded iron formations in rock layers indicate? How did they form?
Banded iron formations are thought to have formed in sea water as the result of oxygen production by photosynthetic cyanobacteria. The oxygen combined with dissolved iron in Earth's oceans to form insoluble iron oxides, which precipitated out, forming a thin layer on the ocean floor.
What is the connection between Eukaryotic plant cells and Prokaryotic photosynthesizers?
The Endosymbiotic Theory suggests that chloroplasts were once free-living prokaryotic photosynthesizes that were engulfed by the ancestor of today's plants.
Write the equation for photosynthesis
6CO2 + 6H2O + light energy = C6H12O6 + 6O2.
What is stored in the glucose molecule? ______________!
energy
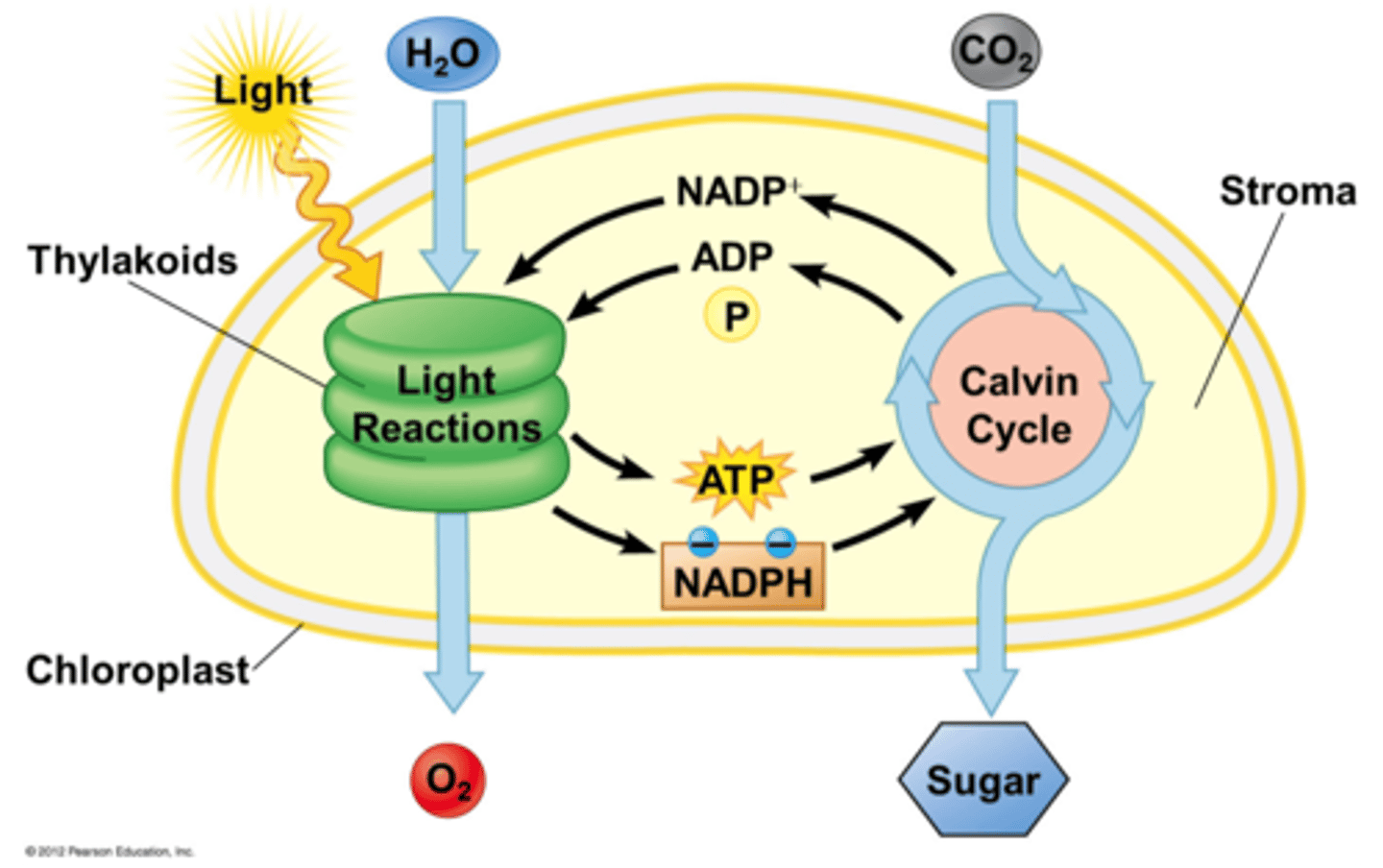
Light Dependant Reaction
Where does the light reaction take place specifically? ___________________________
thylakoid membranes of organelles called chloroplasts.
What is chlorophyll and what does it do?
Chlorophyll is the natural compound present in green plants that gives them their color.
It helps plants to absorb energy from the sun as they undergo the process of photosynthesis
What does light do in the light dependant reactions (what is it used for)?
use light energy to make two molecules needed for the next stage of photosynthesis: the energy storage molecule ATP and the reduced electron carrier NADPH
As the electrons pass through the __________________________ between Photosystem I and II, they use their energy to pump ___________ into the ____________________________________ creating an electrochemical gradient, and making that space very (acidic/basic).
electron transport chain
electrons
thylakoid lumen (interior)
acidic
When the protons are concentrated on one side of the membrane, they pass through the ___________________(name of enzyme) and forms ____________!
ATP synthase
ATP
What is the final electron acceptor in the Electron Transport Chain of the Light Dependant Reaction?
NADP
Where did the electron come from that is added to NADPH (what molecule did it come from originally)?
ETC
What waste product is made during the light dependant reaction? (HINT it is a molecule we need!)
oxygen
Where does the ATP and NADPH go? What is their purpose?
used to make sugars in the next stage of photosynthesis, the Calvin cycle
cyclic photophosphorylation, electrons follow a different, circular path and only ATP (no NADPH) is produced.
Calvin Cycle (Light Independent reaction):
Specifically where does the Light Independent Reaction happen?
stroma of chloroplast
What is the ultimate product formed during the light independent reaction? ______________
glucose (sugar)
Where do the Carbon atoms come from to make this product? _____________
he atmospheric carbon dioxide molecules that are taken in by plants for photosynthesis.
Draw a simple diagram of a chloroplast. Label the thylakoid, thylakoid membrane, and stroma. Label where the light dependant and Calvin Cycle (light independent reactions) are located. Label where Water and CO2 enter the process, and where O2 and Glucose are formed.
TOPIC 3.6 Cellular Respiration
ENDURING UNDERSTANDING: The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.K Describe the processes that allow organisms to use energy stored in biological macromolecules.
ESSENTIAL KNOWLEDGE
ENE-1.K.1 Fermentation and cellular respiration use energy from biological macromolecules to produce ATP. Respiration and fermentation are characteristic of all forms of life.
ENE-1.K.2 Cellular respiration in eukaryotes involves a series of coordinated enzyme-catalyzed reactions that capture energy from biological macromolecules.
ENE-1.K.3 The electron transport chain transfers energy from electrons in a series of coupled reactions that establish an electrochemical gradient across membranes—
a. Electron transport chain reactions occur in chloroplasts, mitochondria, and prokaryotic plasma membranes.
b. In cellular respiration, electrons delivered by NADH and FADH are passed to a series of electron acceptors as they move toward the terminal electron acceptor, oxygen. In photosynthesis, the terminal electron acceptor is NADP+. Aerobic prokaryotes use oxygen as a terminal electron acceptor, while anaerobic prokaryotes use other molecules.
c. The transfer of electrons is accompanied by the formation of a proton gradient across the inner mitochondrial membrane or the internal membrane of chloroplasts, with the membrane(s) separating a region of high proton concentration from a region of low proton concentration. In prokaryotes, the passage of electrons is accompanied by the movement of protons across the plasma membrane.
d. The flow of protons back through membrane-bound ATP synthase by chemiosmosis drives the formation of ATP from ADP and inorganic phosphate. This is known as oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis.
e. In cellular respiration, decoupling oxidative phosphorylation from electron transport generates heat. This heat can be used by endothermic organisms to regulate body temperature
X The names of the specific electron carriers in the electron transport chain are beyond the scope of the AP Exam
LEARNING OBJECTIVE
ENE-1.L Explain how cells obtain energy from biological macromolecules in order to power cellular functions.
ESSENTIAL KNOWLEDGE
ENE-1.L.1 Glycolysis is a biochemical pathway that releases energy in glucose to form ATP from ADP and inorganic phosphate, NADH from NAD+, and pyruvate.
ENE-1.L.2 Pyruvate is transported from the cytosol to the mitochondrion, where further oxidation occurs.
ENE-1.L.3 In the Krebs cycle, carbon dioxide is released from organic intermediates, ATP is synthesized from ADP and inorganic phosphate, and electrons are transferred to the coenzymes NADH and FADH2.
ENE-1.L.4 Electrons extracted in glycolysis and Krebs cycle reactions are transferred by NADH and FADH2 to the electron transport chain in the inner mitochondrial membrane.
ENE-1.L.5 When electrons are transferred between molecules in a sequence of reactions as they pass through the ETC, an electrochemical gradient of protons (hydrogen ions) across the inner mitochondrial membrane is established.
ENE-1.L.6 Fermentation allows glycolysis to proceed in the absence of oxygen and produces organic molecules, including alcohol and lactic acid, as waste products.
ENE-1.L.7 The conversion of ATP to ADP releases energy, which is used to power many metabolic processes.
X Specific steps, names of enzymes, and intermediates of the pathways for these processes is beyond the scope of the AP Exam
X Memorization of the steps in glycolysis and the Krebs cycle, and of the structure of molecules and the names of enzymes involved, are beyond the scope of the AP Exam
Cellular Respiration
Draw a simple mitochondria. Label the location of Glycolysis, the Krebs cycle, and the Electron Transport Chain.
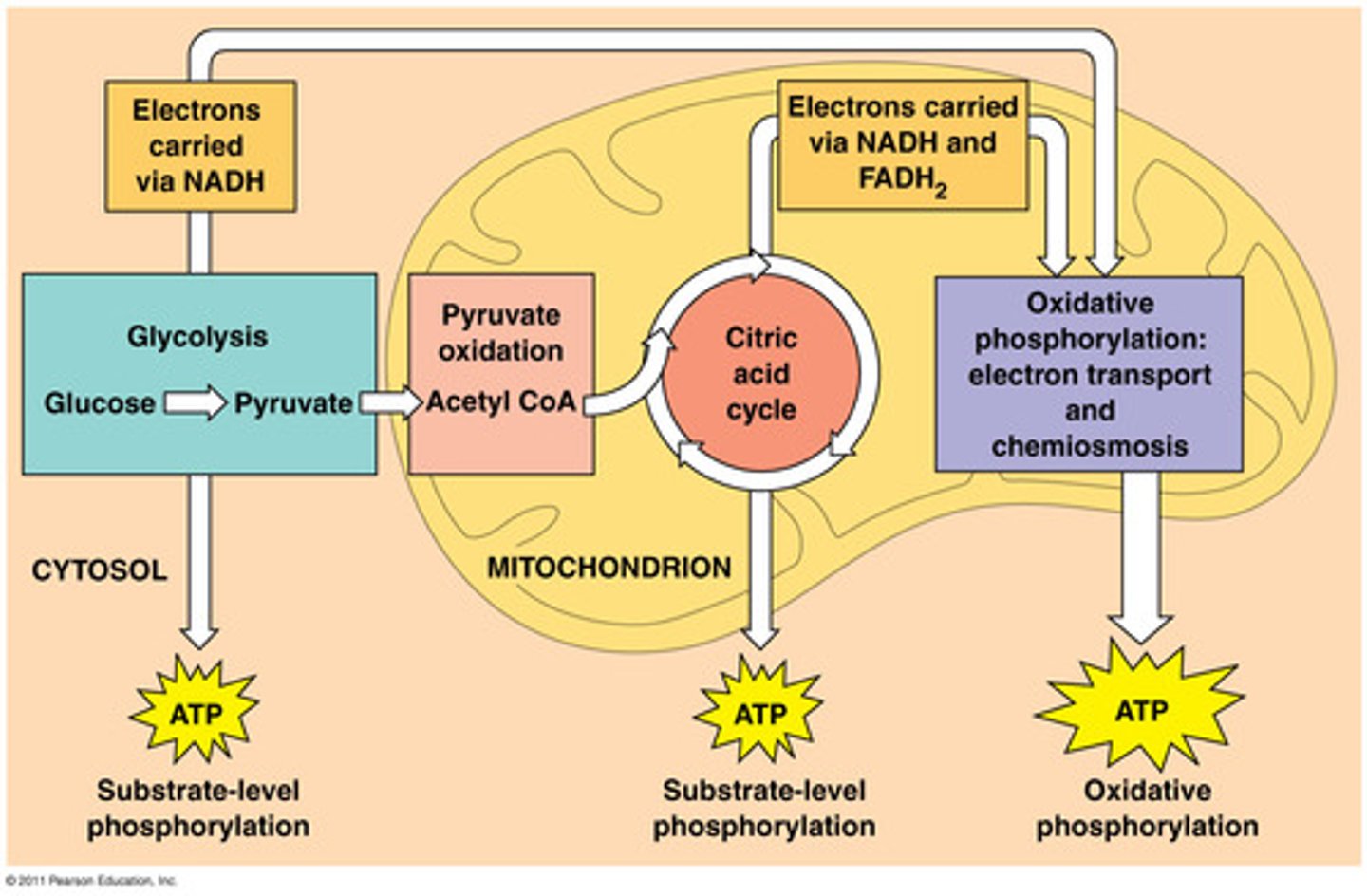
Fermentation does not require _________ and is categorized as ____________ cellular respiration.
oxygen
anaerobic
What type of fermentation do yeast do? ______________What is the product? ____________
alcoholic fermentation
ethanol
What type of fermentation do we do? ______________ What is the product? _____________
lactic acid
lactic
Glycolysis starts with this molecule...
glucose
and makes what three useful molecules...
ATP, NADH, pyruvate
Where does NADH go? What does it do?
NADH carries high energy electrons to the electron transport chain
Where does pyruvate go (for aerobic respiration)? What is its purpose?
pyruvate goes on to the keens cycle where are t is further oxidized, making FADH and NADH which goes the the ETC
Glycolysis is considered "highly conserved" across all domains of life. What does this mean, and what does it imply about the evolution of the process?
this means that members of all domains of life do glycolysis, suggesting that glycolysis evolved as far back as the last universal common ancestor, and was passed down to every form of life being "conserved " throughout the billions of generations between now and then
How does the location of Glycolysis support this theory?
glycolysis happens in the cytoplasm of all cells which support the idea that large prokaryote cell engulfed an aerobic prokaryote cell
What is the general purpose of the Krebs Cycle?
to further oxidize pyruvate, generate more high energy molecules
What three important molecules are made in the Krebs Cycle?
NADH, ATP, FADH
Where do NADH2 and FADH go next? What are they carrying?
they are carrying high energy electrons to the ETC
What waste product is produced during the Krebs Cycle? (HINT its a molecule Plants need!)
carbon dioxide
Electron transport chain.
As electrons are transferred down the ETC _________ are pumped into the _____________________________ which establishes an electrochemical gradient, and makes that space very (acidic/basic).
protons
intermembrane space
acidic
What is the final electron acceptor at the end of the ETC?
oxygen
What 'waste' molecule is produced?
CO2 and water
Protons flow back across the membrane through this enzyme...
enzyme ATP synthase
In a process called.... (what type of membrane transport)
chemiosmosis
atp synthase
Which makes ATP by joining ATP and inorganic phosphate in a process called...
ATP Synthase through oxidative phosphorylation
Go back to your Mitochondria diagram on the previous page. Label where O2 and Glucose substrates are used, and where CO2 and H2O Products are produced.
In very simple terms, what does 'phosphorylation' mean? ____________________
the chemical addition of a phosphoryl group (PO3-) to an organic molecule.
Process: Glycolysis, Krebs Cycle,
Electron Transport Chain
Type of phosphorylation? (substrate level or oxidative)
How many net ATP are made per glucose?
Glycolysis:
Krebs Cycle:
Electron Transport Chain:
Some prokaryotes also have Electron Transport Chains - but do not have mitochondria or chloroplasts. How do they establish a proton gradient with which to make ATP?
During Fermentation, pyruvate (end product of ________________) is converted to Lactic Acid or Alcohol by taking hydrogens and electrons from ______________. How does this allow the process of Glycolysis to continue?
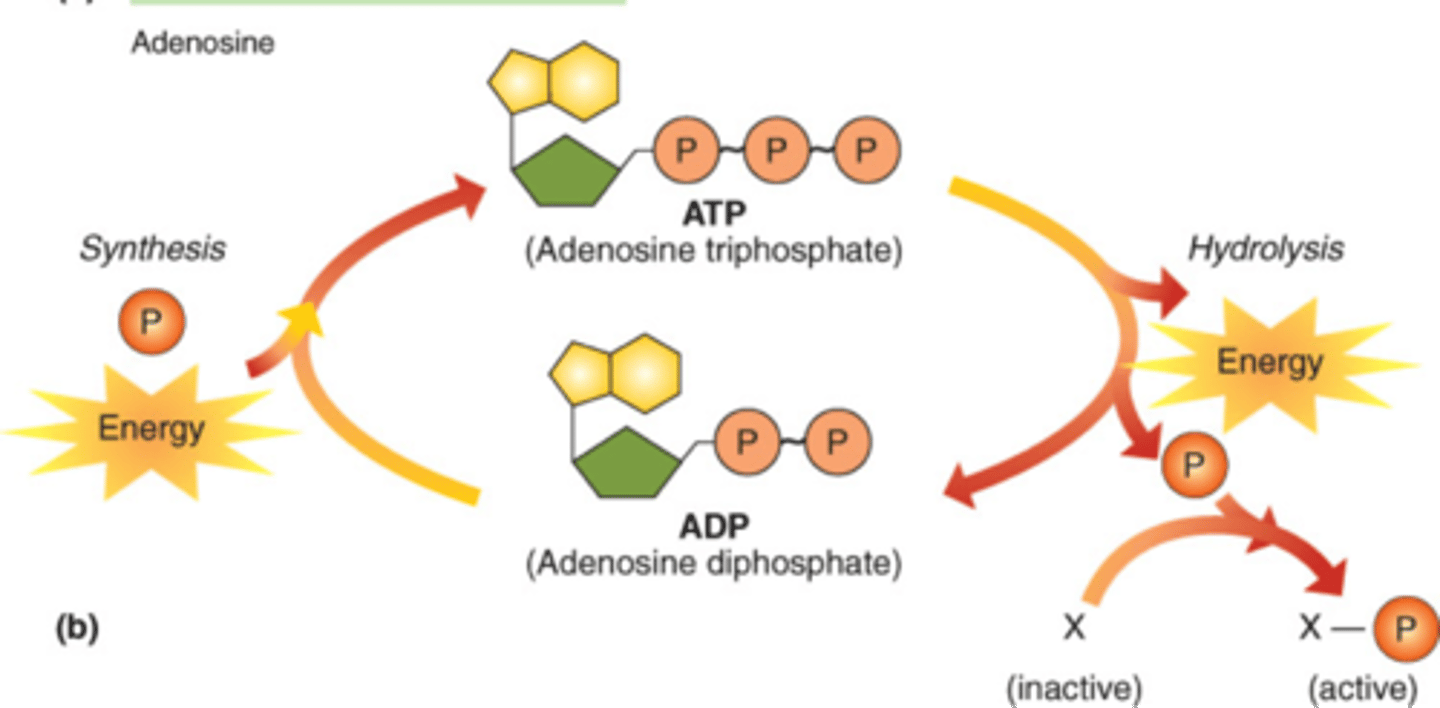
What is this molecule? __________
Draw an arrow to the bond that is broken that releases energy to power cellular processes
ATP
In your own words, how do endotherms generate body heat?
TOPIC 3.7 Fitness
ENDURING UNDERSTANDING: Naturally occurring diversity among and between components within biological systems affects interactions with the environment.
LEARNING OBJECTIVE
SYI-3.A Explain the connection between variation in the number and types of molecules within cells to the ability of the organism to survive and/or reproduce in different environments.
ESSENTIAL KNOWLEDGE
SYI-3.A.1 Variation at the molecular level provides organisms with the ability to respond to a variety of environmental stimuli.
SYI-3.A.2 Variation in the number and types of molecules within cells provides organisms a greater ability to survive and/or reproduce in different environments.
ILLUSTRATIVE EXAMPLES § Different types of phospholipids in cell membranes allow the organism flexibility to adapt to different environmental temperatures. § Different types of hemoglobin maximize oxygen absorption in organisms at different developmental stages. § Different chlorophylls give the plant greater flexibility to exploit/ absorb incoming wavelengths of light for photosynthesis.
Phospholipids in Cell Membranes
In cold temperatures, phospholipid bilayers with saturated fatty acid tails are more rigid, while phospholipid bilayers with unsaturated fatty acid tails maintain the fluidity and flexibility that cells require.
During different times of the year, some plants (like Winter Wheat) can change the concentration of unsaturated phospholipids.
The diagram shows the composition of Winter Wheat phospholipids in the summer. Draw what you would expect to see in the winter.
Plants have two forms of chlorophyll, a and b. What wavelengths do each absorb?.
Chlorophyll a - _______ & ________
Chlorophyll b - _______ & ________
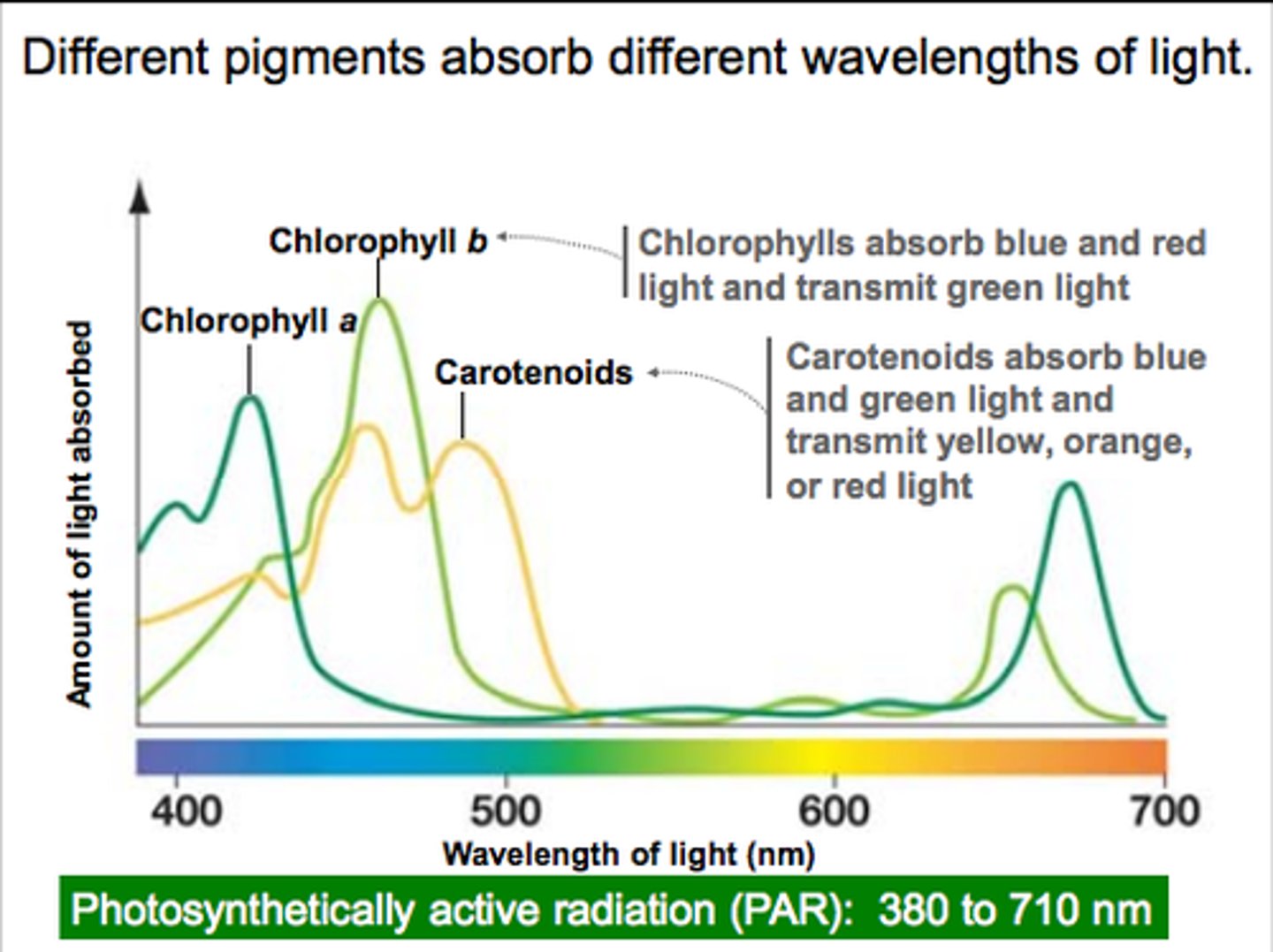
What is the adaptive advantage of having two forms of chlorophyll that absorb different light wavelengths?