Amount of Substance 1.2
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
What is Avagadros number?
6.022× 10 ^ 23
What is the equation to calculate the number of particles in a substance?
Number of particles=Avagadros number x Number of moles
What is the equation to find the number of moles?
Number of moles= mass/Mr or Ar
What is the equation to calculate the number of moles of a substance in a solution?
Number of moles= concentration/volume
How do you convert cm³ into dm³
Divide by 1000
What is the ideal gas equation?
pV=nRT
P pressure (Pa)
V volume (m³)
n number of moles
R gas constant 8.31 JK ^-1 mol ^-1
T temperature (Kelvins+273)
What are the standard conditions?
Pressure is 100KPa
Temperature 298 K
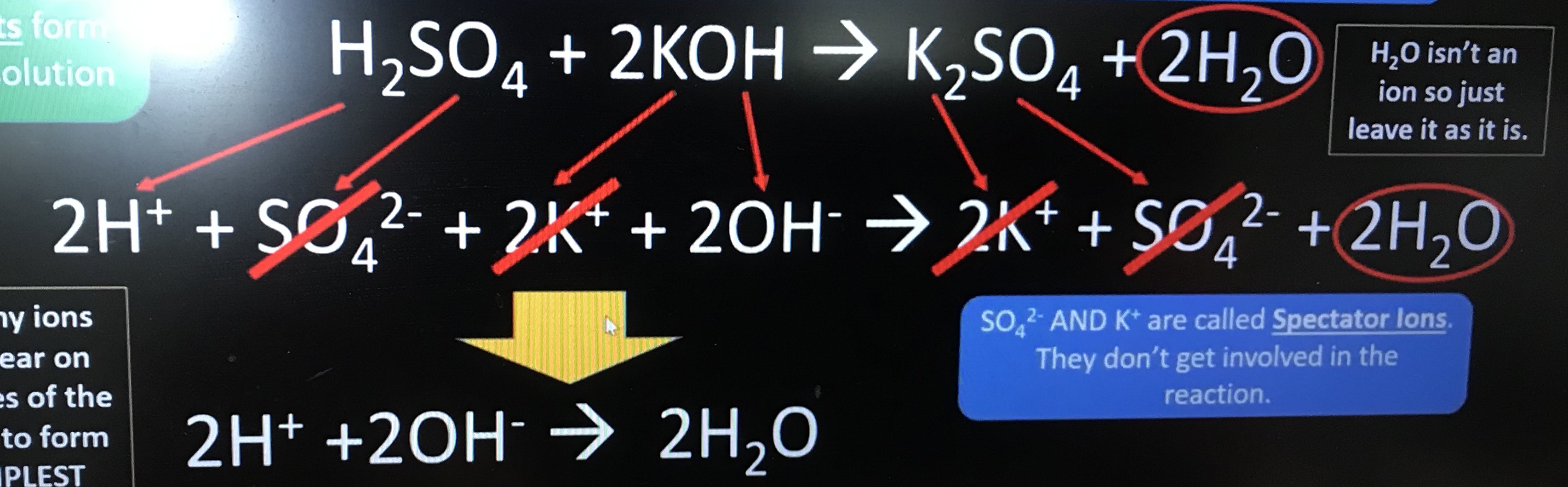
Give the full ionic equation of reaction of sulfuric acid and potassium hydroxide
What range do your results need to be in order to be concordant?
0.1cm³
Definition of Empirical formula
The simplest whole number ratio of elements in a compound
What is the equation for percentage yield?
Percentage yield= actual yield/ theoretical yield X 100
What is the equation for atom economy?
Atom economy = molecular mass of desired product / sum of molecular masses of all reactants or products X 100