Ionic and Covalent Bonds
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
ionic bond (definition)
bond formed when one or more electrons are transfered from one atom to another
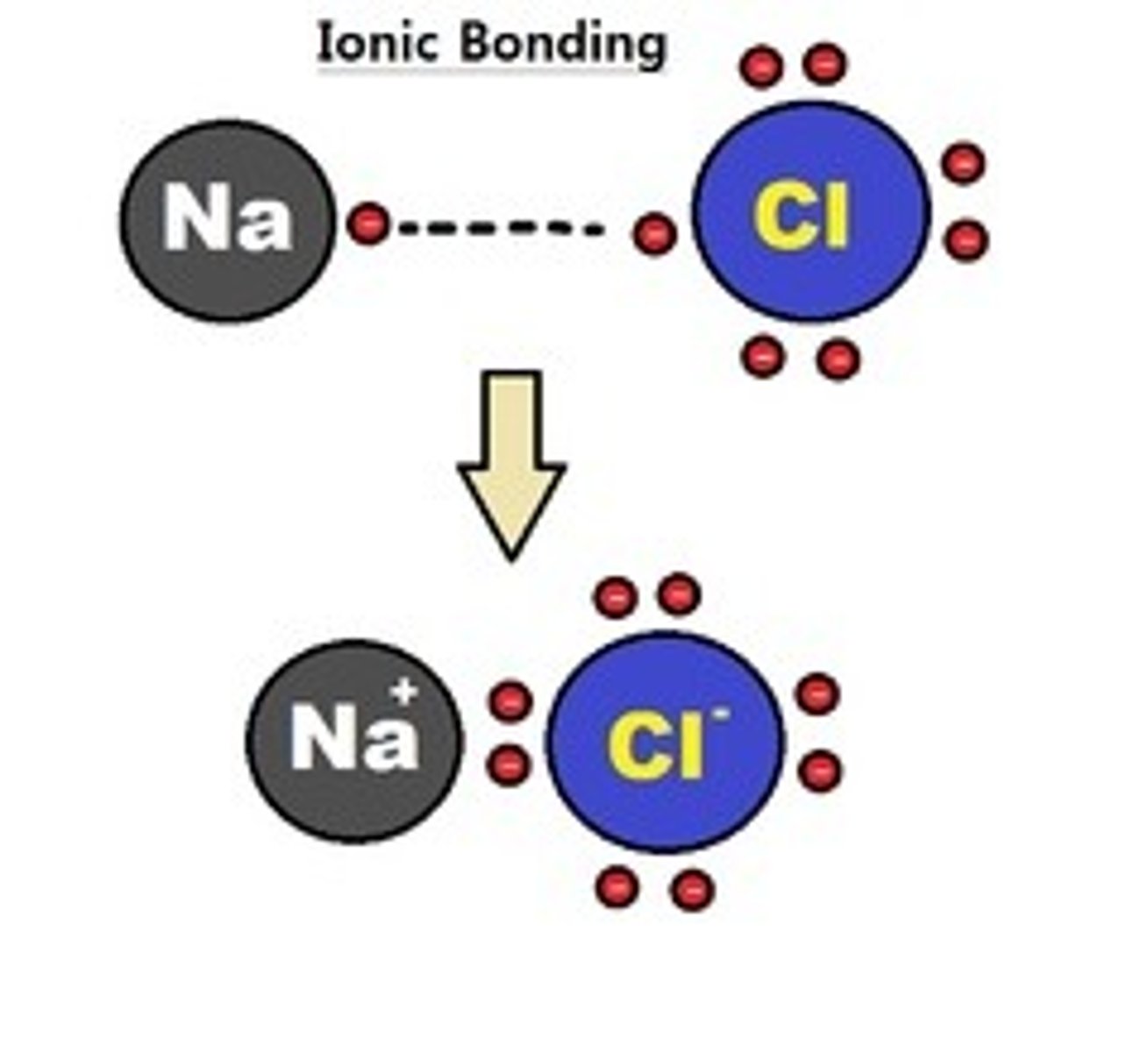
ionic bond (description)
a chemical bond resulting from the attraction between oppositely charged ions.
ionic bond (types of elements)
metals with nonmetals
cation
a positively charged ion

anion
a negatively charged ion
group 1 (charge formed)
1+
group 2 (charge formed)
2+
group 7 (charge formed)
1-
group 6 (charged formed)
2-
covalent bond (definition)
a chemical bond that involves sharing a pair of electrons between atoms in a molecule
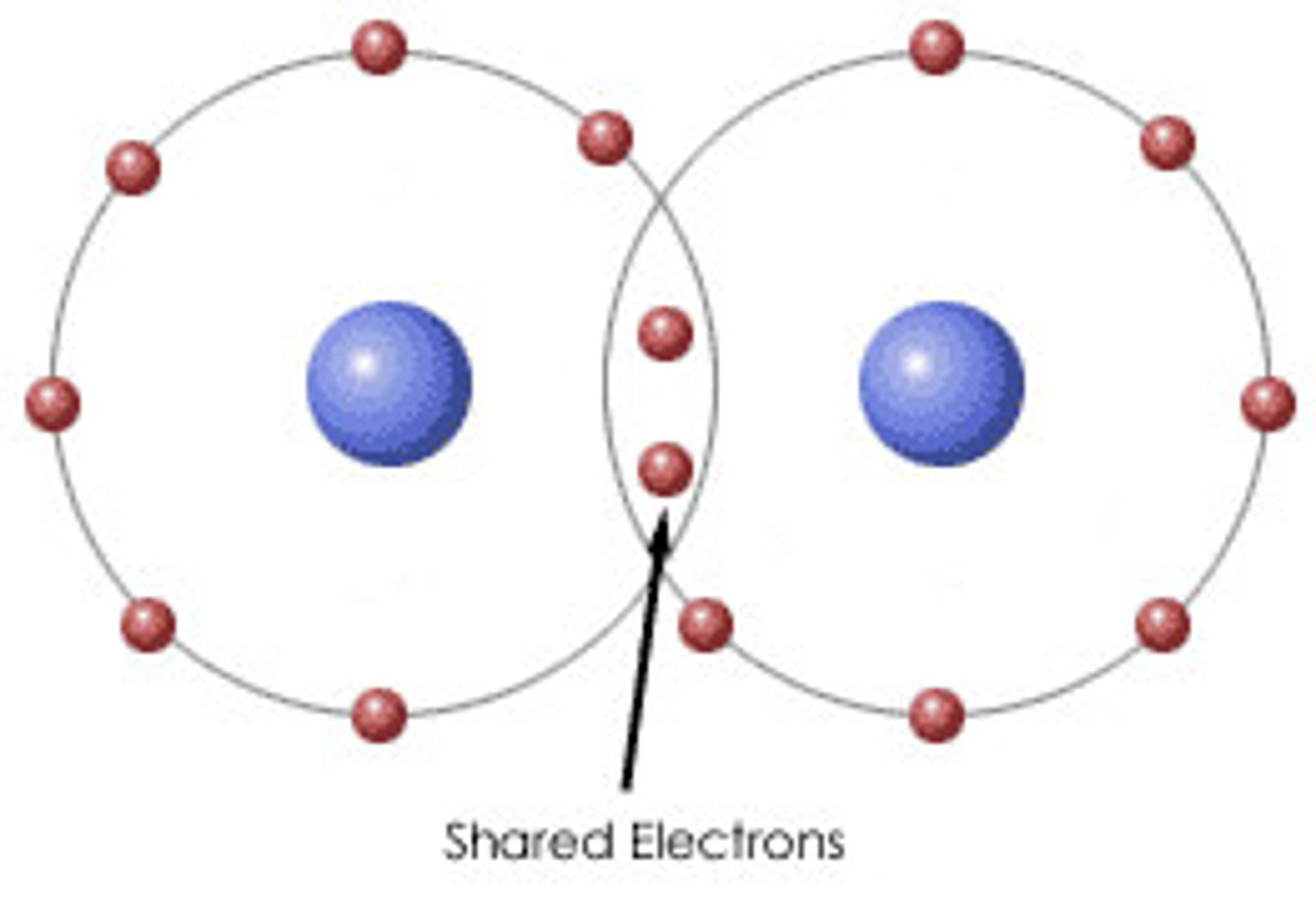
covalent bond (types of elements)
nonmetals bonding together
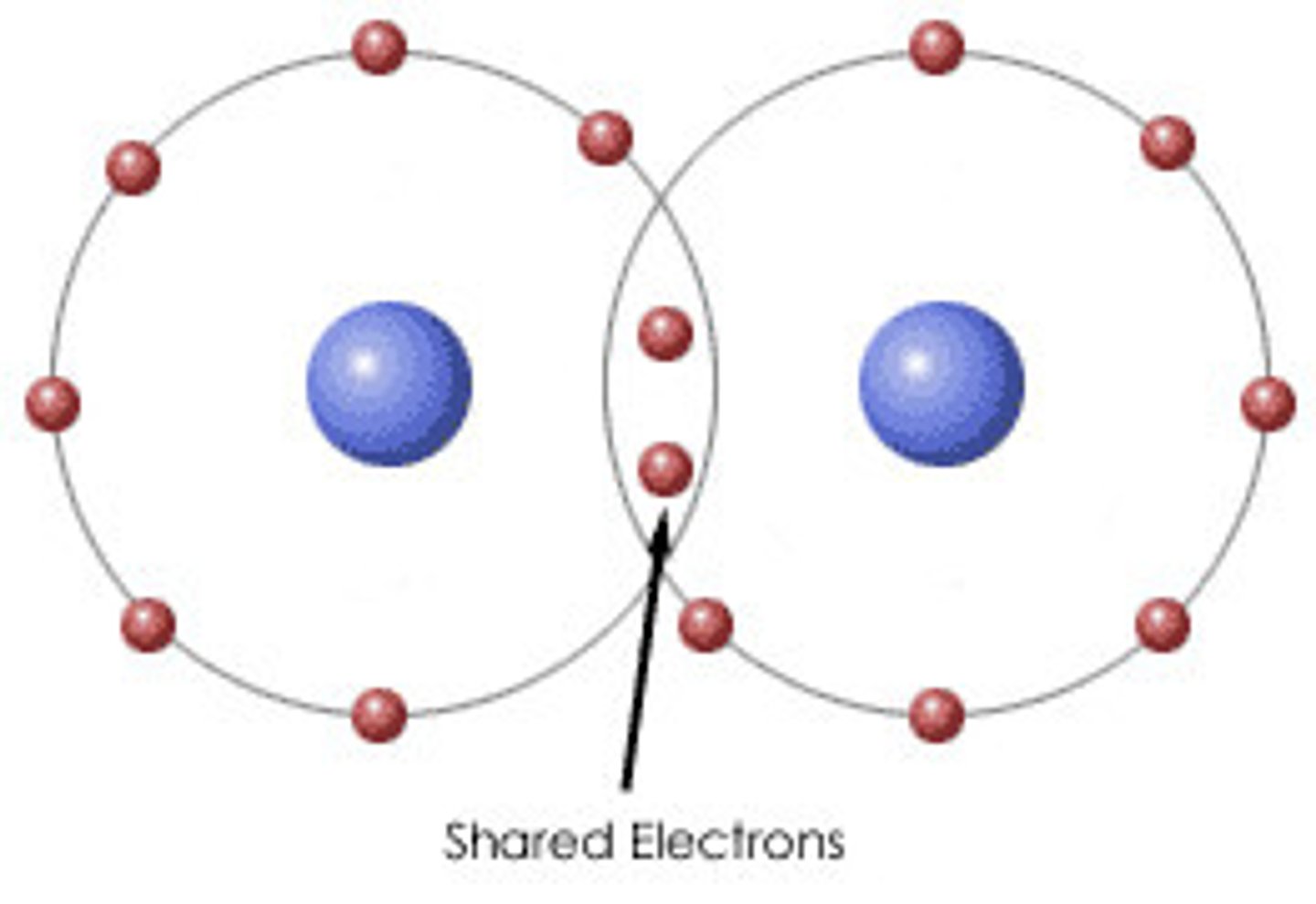
molecule
smallest unit of a covalent compound, two or more atoms held together by covalent bonds
octet rule
atoms react by gaining or losing electrons so as to acquire the stable electron structure of a noble gas, usually eight valence electrons

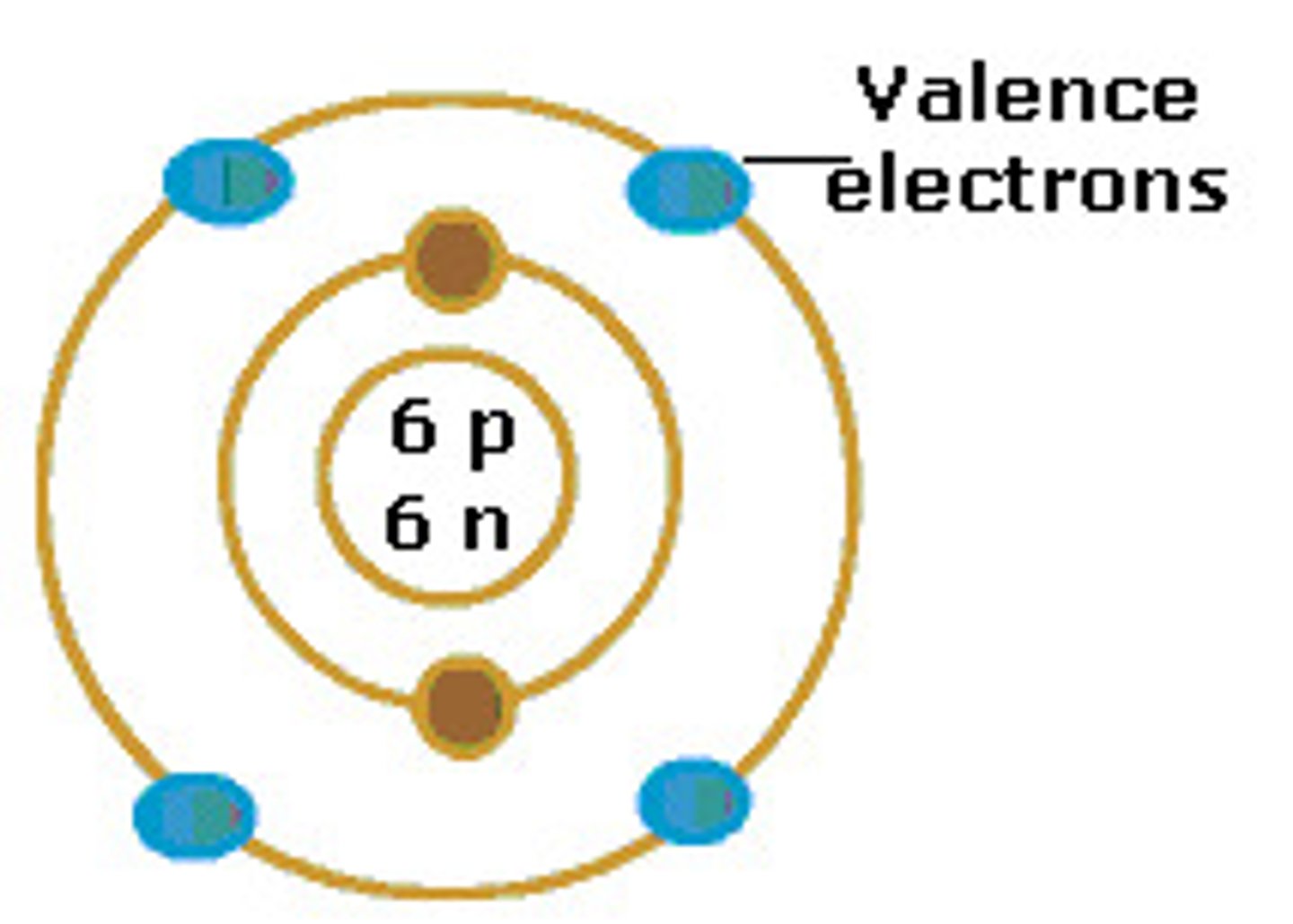
valence electron
Electrons on the outermost energy level of an atom
compound
A substance made up of two or more different elements joined by chemical bonds
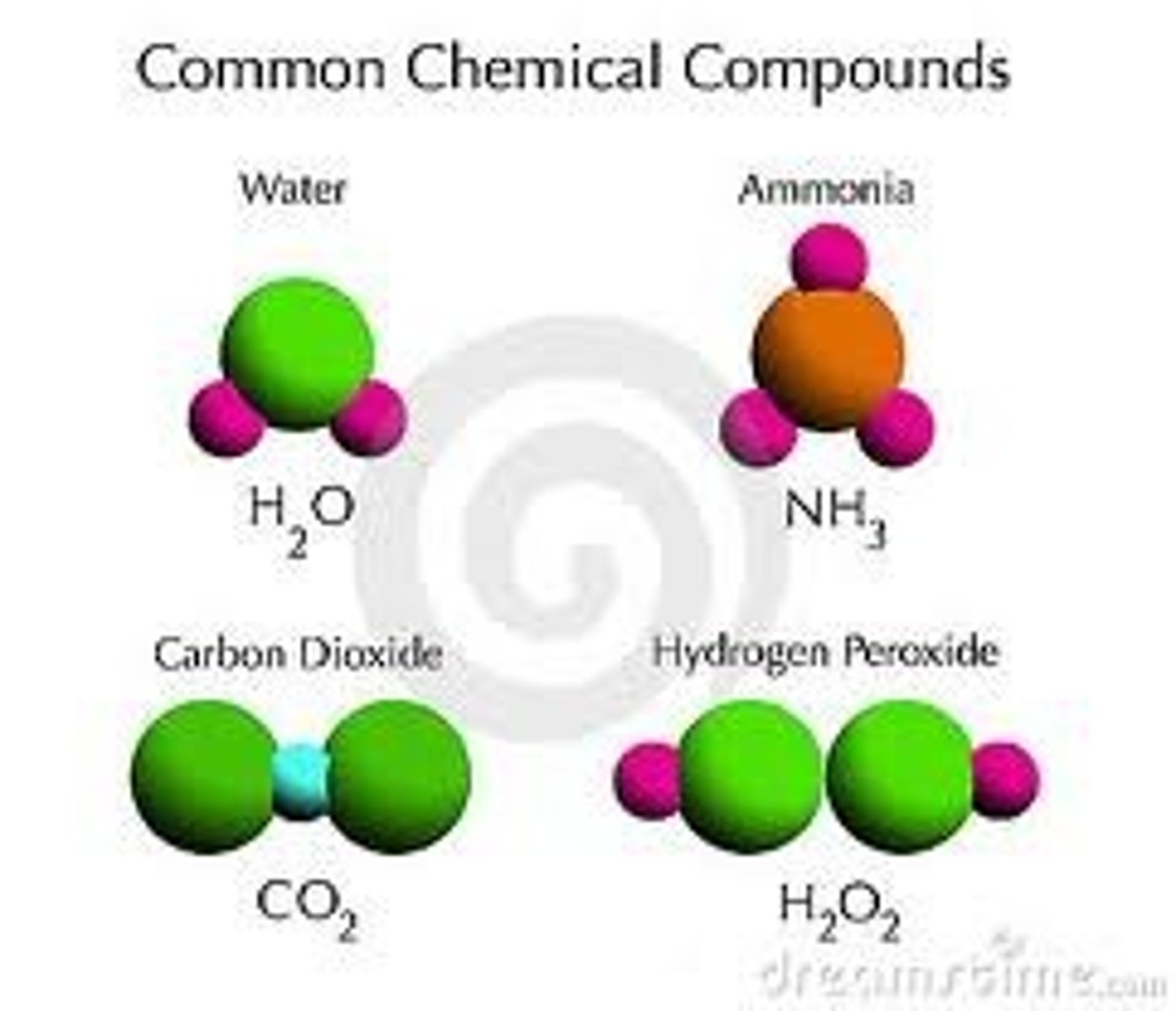
chemical bond
the force that holds two atoms together
ion
A charged atom
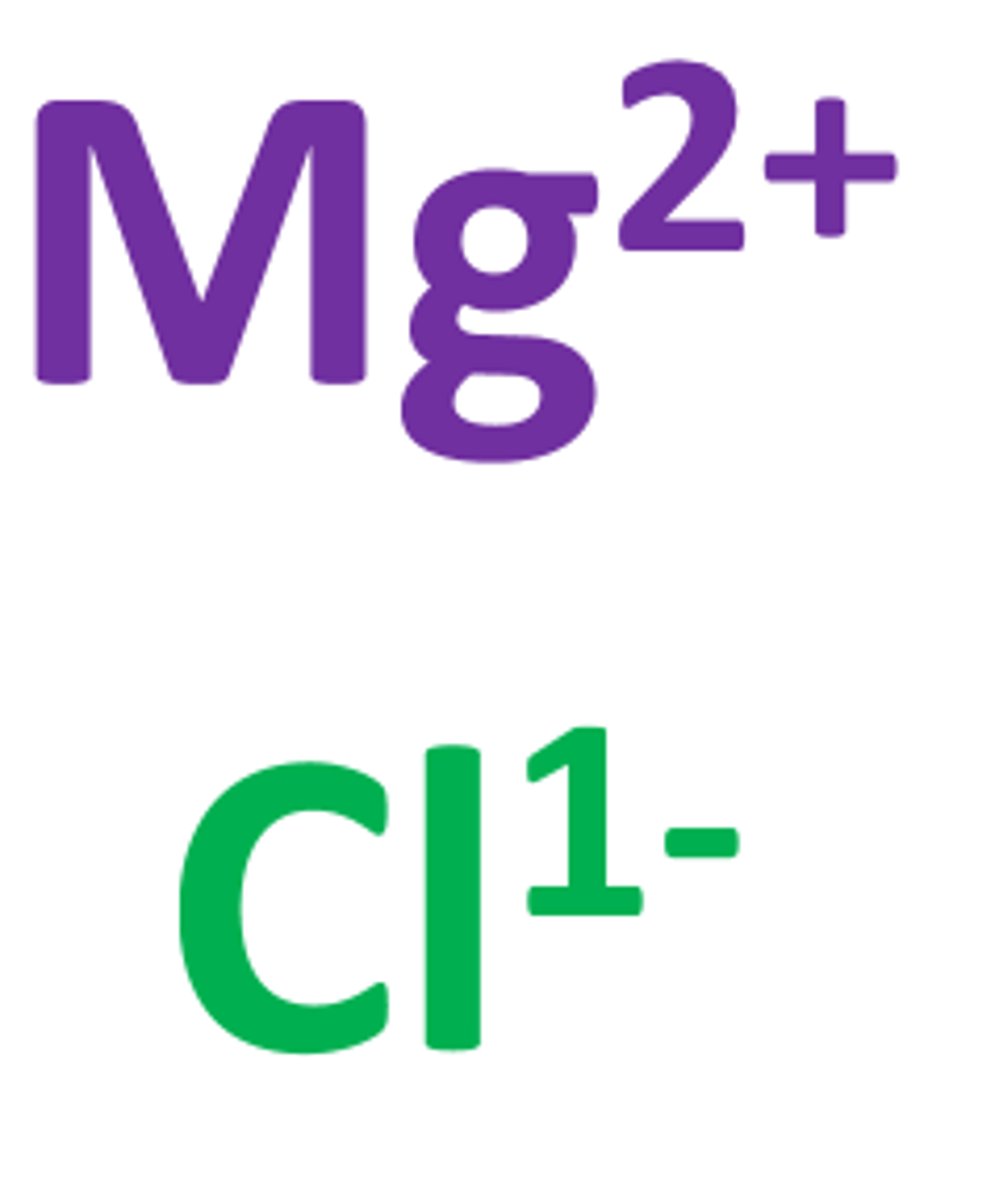
ionic bond
Formed when one or more electrons are transferred from one atom to another
covalent bond
A chemical bond formed when two atoms share electrons
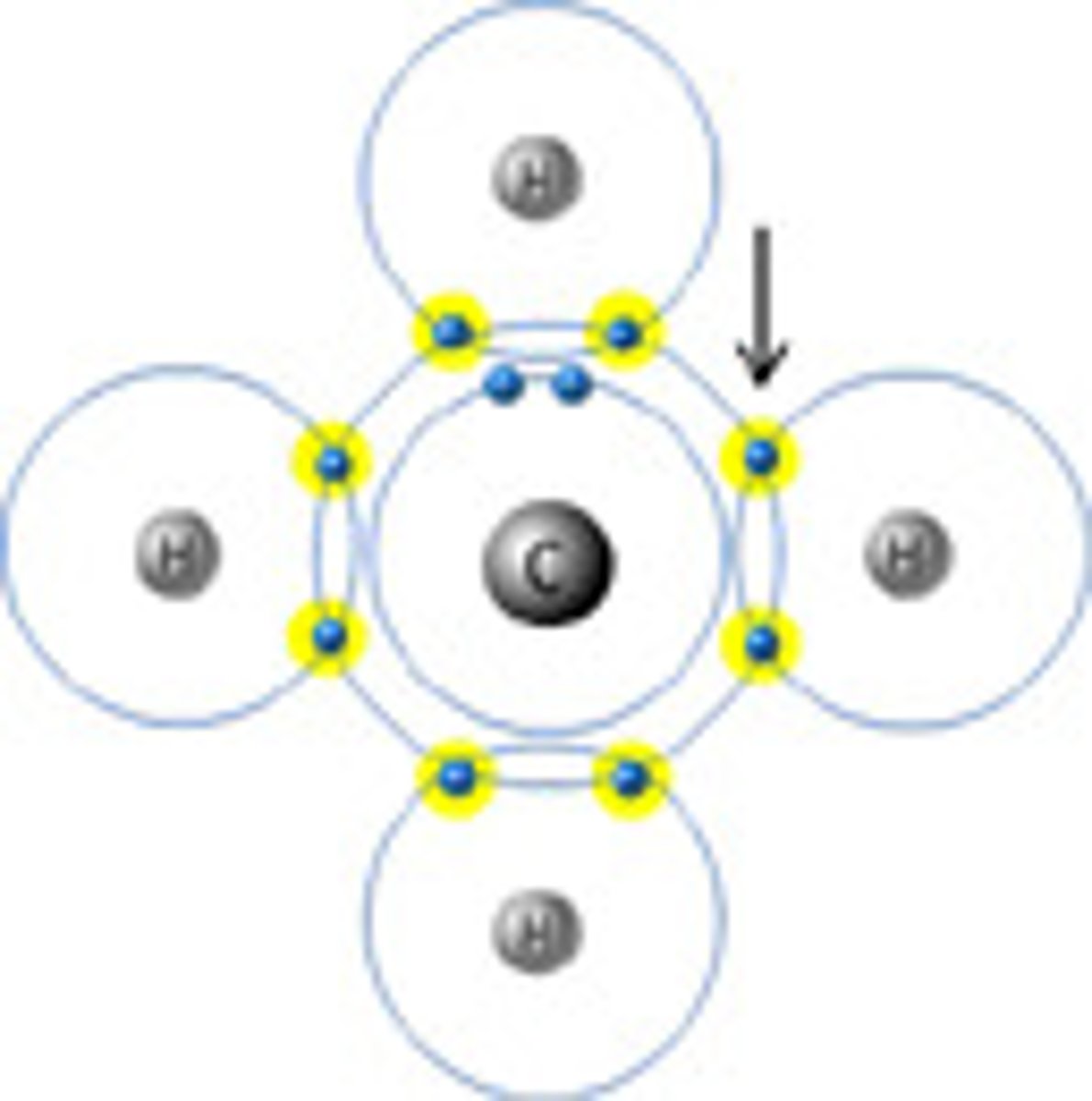
Proton
A charge of +1, a mass = 1 a.m.u. and is located in the nucleus
Electron
A subatomic particle that orbits the nucleus and has a charge of -1. The electron has a negligible mass and is often denoted by the symbol e-.
Neutron
- uncharged subatomic particles in the atom
- located in the nucleus