OCR (A) A level biology - Enzymes
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
What are enzymes?
Tertiary structure globular proteins which act as biological catalysts to speed up the rate of reactions
Anabolic meaning
Chemical reactions required for growth, (building up) reactions
Catabolic meaning
Chemical reacts for breaking down organic molecules
What is meant by metabolism?
Sum of all chemical reactions in the body, can only happen as a result of enzymes
What is meant by the V-Max?
Max rate at which and enzyme can increase the rate of reaction
Specificity of the enzymes meaning
Each enzyme catalyses one biochemical reaction
What must occur for a reaction to happen?
-Molecules need to collide in the correct orientation
-Activation energy must be met (minimum amount of energy required for a reaction to happen)
What is the active site?
Area within the tertiary structure of the enzyme that is complimentary to the shape of a specific substrate molecule
What occurs when the substrate binds to the active site?
An enzyme-substrate complex is formed. The substrate reacts and the products are formed in an enzyme-product complex . The products are then released, leaving the enzyme unchanged and able to take part in subsequent reactions
Lock and key theory
Enzyme is like a lock, substrate is the key
This model suggest that the enzyme active site is a fixed shape, due to collisions the substrate can collide and attach to the enzyme.
R groups within the active site interact with substrate, forming temporary bonds. These put strain on the bonds within substate, so they can be broken down more easily
Induced fit theory
Active site of an enzyme changes slightly as substrate binds, moulding to fit
Weak interactions between enzyme and substrate rapidly induce changes in enzymes tertiary structure, straining the substrate. This weakens bonds in the substrate and therefor lower the activation energy
What are intracellular enzymes?
Enzymes within the cell
Catalase is an intracellular enzyme, catalase breaks down hydrogen peroxide into oxygen and water quickly therefore preventing its accumulation.
(found in both animals and plants)
What are extracellular enzymes?
Enzymes released from cells to break down larger nutrients into smaller molecules (eg in digestive system so nutrients are small enough to be absorbed into bloodstream, they are then transported around the body to be used as substrates for reactions)
In fungi, they work outside the body
Explain the digestion of starch (extracellular enzyme)
1. Enzyme amylase produced in salivary glands and pancreas breaks down starch into maltose (disaccharide)
2. Enzyme maltase breaks down maltose into glucose (monosaccharide)
Glucose now small enough to be absorbed by cells and subsequently absorbed into bloodstream
Explain the digestion of proteins
Trypsin is a type of protease (enzyme that breaks down proteins)
Trypsin is produced in the pancreas, released with the pancreatic juice into small intestines where it acts on proteins
Amino acid produced are absorbed by cells lining digestive system then subsequently into bloodstream
(extracellular enzymes)
What are extracellular enzymes?
Enzymes catalysing chemical reactions which take place outside of the cell
Explain how catabolism and anabolism are related to metabolism (3)
Catabolism is the breaking down of molecules (1), anabolism is the building of molecules (1)
Metabolism is the sum of all chemical reactions, chemical reactions involve building up and breaking down molecules (1)
Explain the effect of temperature of enzyme activity
-Increase in temp results in increased kinetic energy of particles, particles move faster and there are more successful collisions between substrate and enzyme. This leads to an increased rate of reaction
What is meant by the temperature coefficient?
Q10, a measure of how much the rate of reaction increases with a 10 degrees celsius increase.
In enzyme-controlled reactions this is usually taken as two meaning:
Q10 = 2 (rate doubles with a 10 degrees increase)
Other example:
Q10 = 3 (rate triples with a 10 degrees increase)
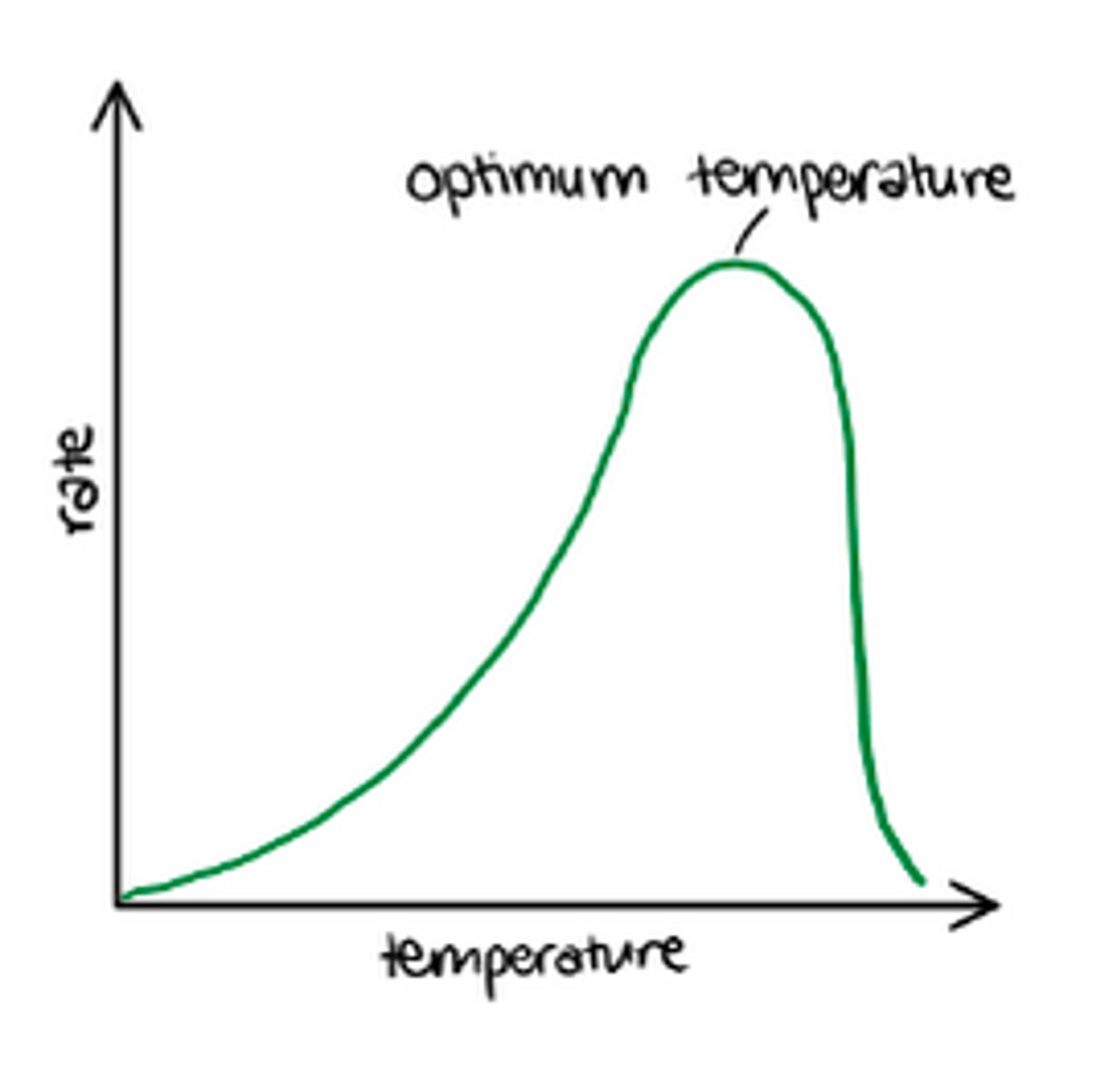
Describe the effect of too low or too high a temperature on an enzyme
Too high: enzyme denatures. As enzymes are proteins, their structure is affected by extreme temperatures as the bonds holding the protein together vibrates more. The vibrations increase with temperature until the bonds strain and break.
Breaking of these bonds results in a change in tertiary structure. This change in active site is the denature, no longer complimentary to substrate
Too low: enzyme is inactive (NOT DENATURED).
What is meant by the optimum temperature?
Temperature at which the enzyme has the highest rate of activity.
Optimum temperature varies with different enzymes.
Enzymes in the human body usually optimum at 40 degrees celsius, whereas enzymes in hot springs are much higher.
Once enzymes have denatured above their optimum, the temperature coefficient has no effect
How are enzymes adapted to living in cold environments?
More flexible structures (particularly active sites), making them less stable than enzymes that work at higher temperatures.
Smaller temperature change will denature them
How are enzymes adapted to living in hot environments?
Thermophiles are more stable than other enzymes due to increased number of bonds (particularly H and S bond) in their tertiary structures.
Shape of these enzymes are more resistance to change as temperature rises
What is meant by pH?
Concentration of hydrogen ions in a solution.
A low pH = High hydrogen proton concentration
Acidic
A high pH = Low hydrogen proton concentration
Alkaline
pH affects ionic and hydrogen bonds
What is meant by optimum pH?
Active site will only be in the right shape at a certain hydrogen ion concentration.
When pH changes, becoming more acidic or more alkaline the hydrogen ion concentration is no longer at its certain optimum amount, active site is altered
Too low or too high = denatured
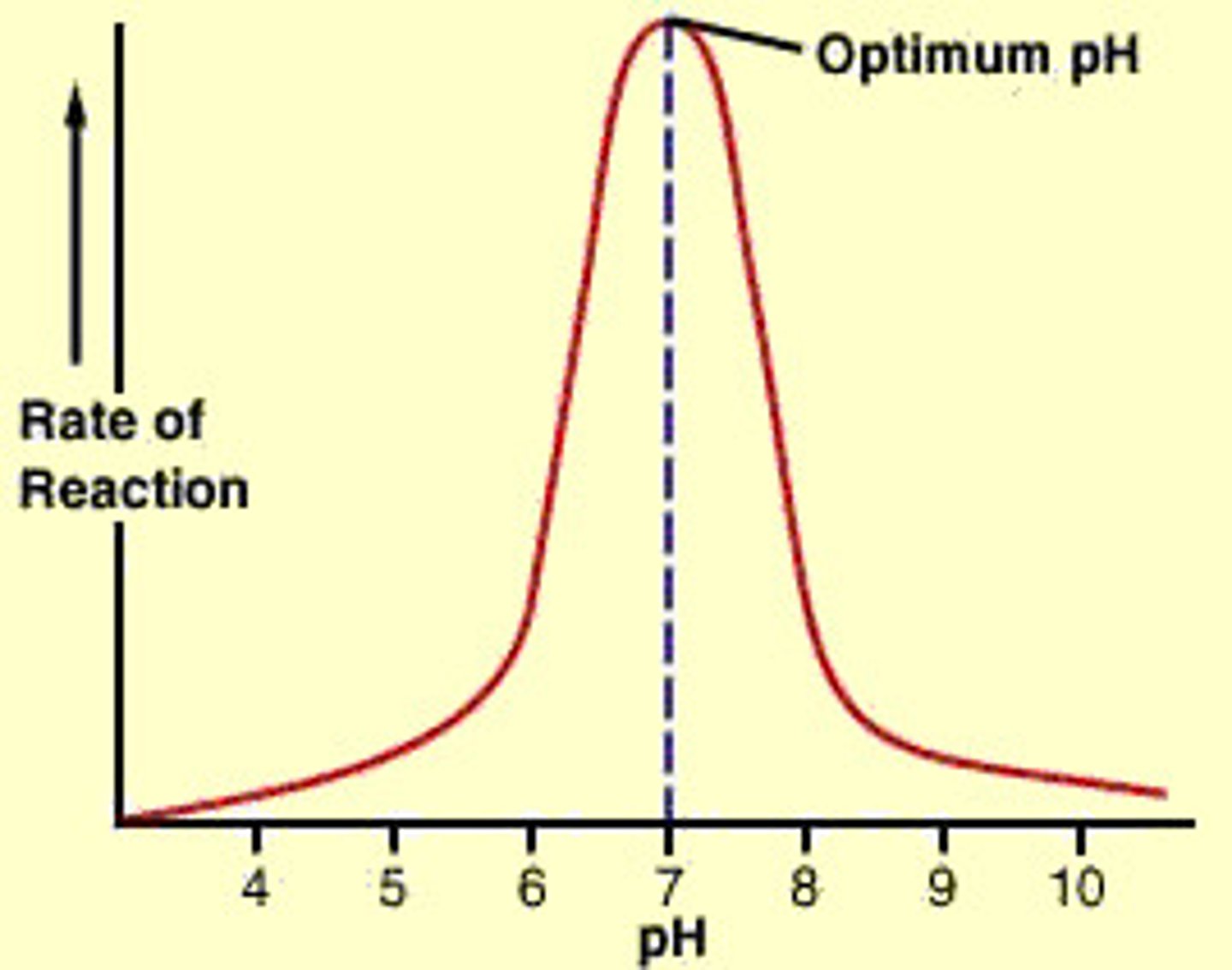
What is meant by renaturation?
pH returns to its optimum, protein changes shape back to normal and the enzyme can resume its role
How does the concentration of hydrogen ions affect the structure of the enzyme (protein)
Hydrogen ions interact with polar/charged R groups in the protein.
Changing the concentration of hydrogen ions therefor changes the degree of the interaction.
The less H+ present (high pH), the less able the R groups are able to interact with each other. This leads to bonds breaking, changing the shape of the protein as it unfolds
High/low vice versa
a) Amylase (salivary gland) pH
b) Pepsin pH
c) Trypsin/maltase/lipase/amylase (small intestine) pH
a) 7-8
b) 1-2
c) 8
How does substrate and enzyme concentration effect enzyme activity?
Substrate: Increased number of substrate particles leads to a higher collision rate with the active sites, rate of reaction increases due to more enzyme-substrate complexes
Enzyme: Increased enzyme concentration increases the number of active sites in a particular area, number of enzyme-substrate complexes increase. Rate of reaction increases
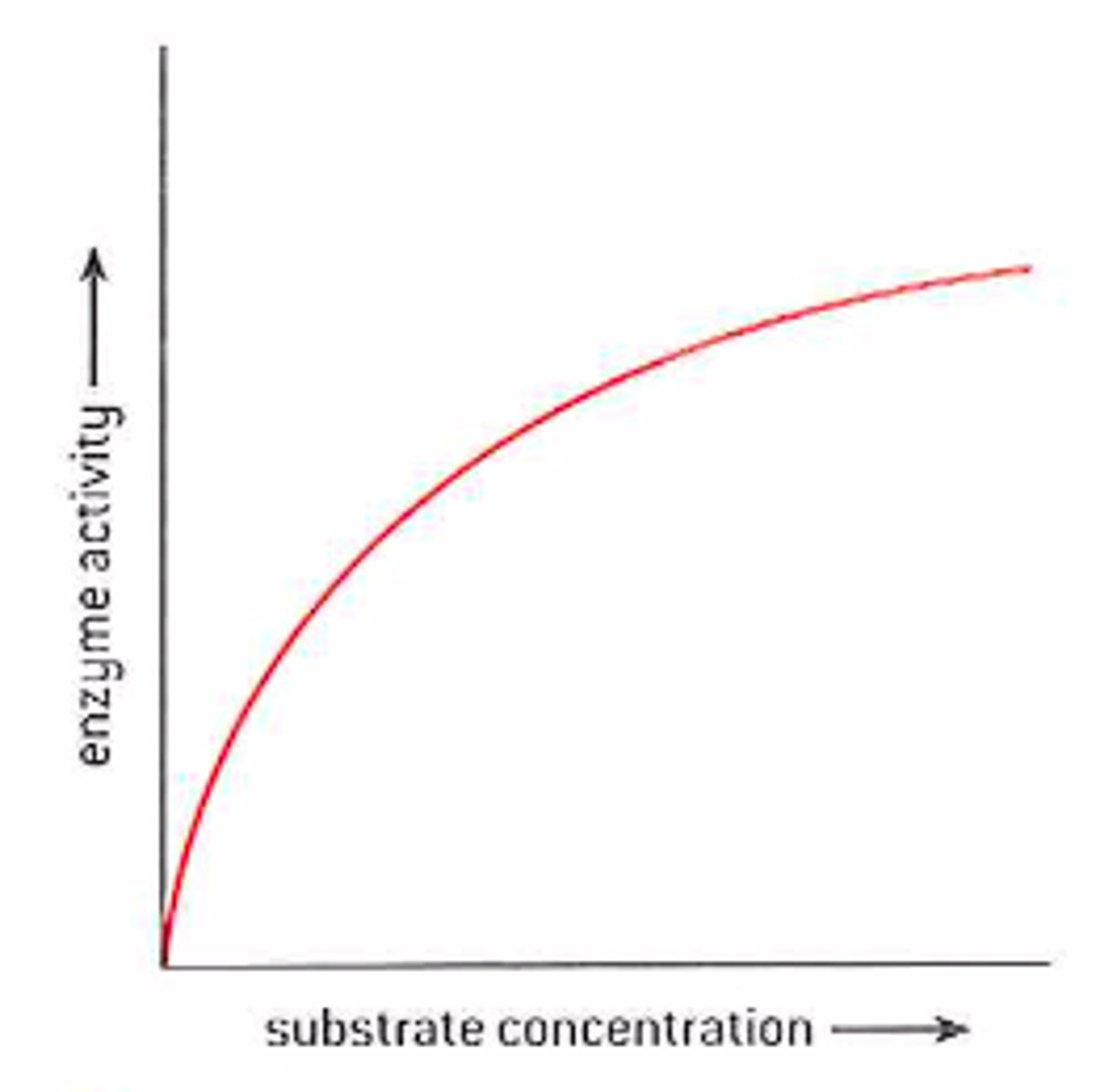
How do you increase the rate of reaction once V-max has been reached
-Increase temperature
-Add more enzyme
(v-max in graph is the plateau)
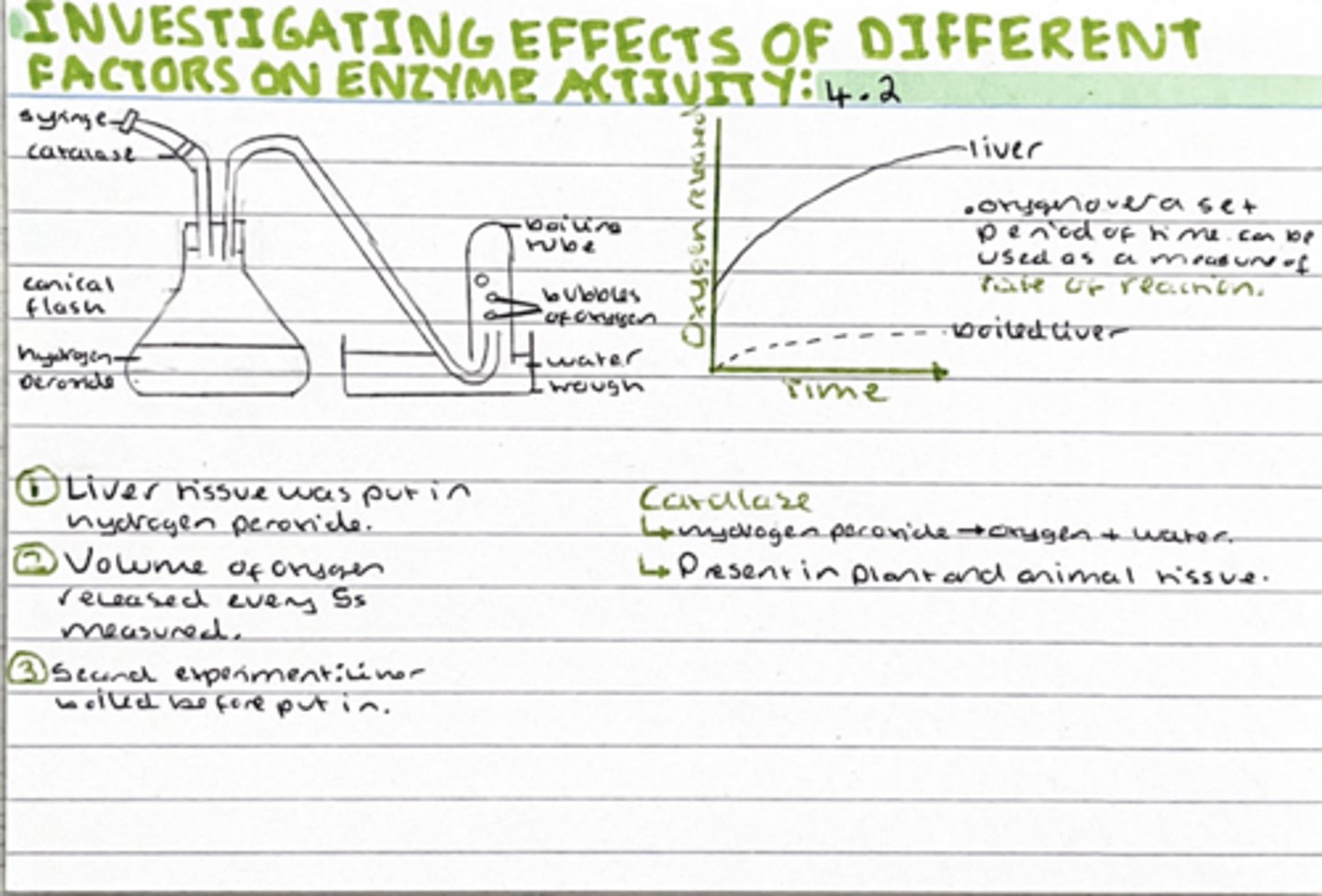
Investigation into the effects of different factors on enzyme activity
a) Explain why the student used liver tissue
Liver tissue into hydrogen peroxide, volume of oxygen released every 5 seconds was measured.
a) contains catalase
Catalase is the enzyme responsible for the breakdown of hydrogen peroxide
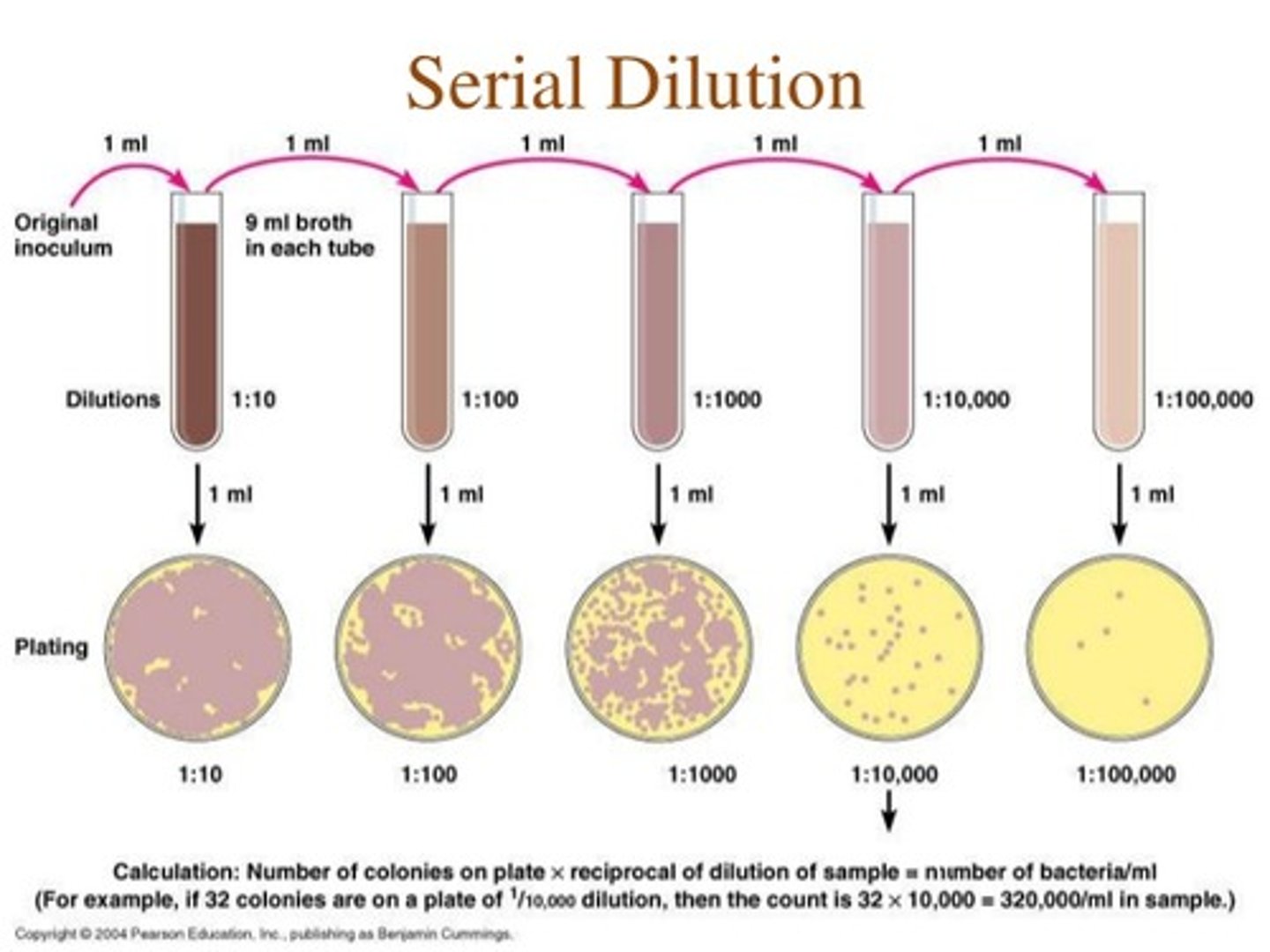
What are serial dilutions?
Step by step dilution of a substance in solution, used to produce a range of concentrations.
Set up by adding 1ml of stock solution into 9ml distilled water. Continue by using 1ml stock from previous tube, add to next tube of 9ml distilled water and so on
10% to 1% to 0.1% tube to tube
How can we use catalase to investigate the effect of different concentrations of catalase on the break down of hydrogen peroxide?
Catalase-rich tissues such as liver ground down into a solution (solution will contain catalase), serial dilution of this solution produces a range of catalase concentrations. Add equal volumes of hydrogen peroxide to each and connect bung. Measure the volume of oxygen produced
Enzymes with very low optimum temperatures tend to have quite flexible structures. Using your knowledge of collision theory, explain why this flexibility is necessary.
At low temperatures kinetic energy is low, substrates/enzymes move more slowly so there are fewer collisions. Increased flexibility of the active site increases the chances of successful collisions.
How to calculate temperature coefficient from a graph
A) calculate the temperature coefficient: 20 degrees, rate of reaction 5 units
30 degrees, rate of reaction 15 units
15/5 = 3a

What is meant by an apoenzyme?
Precursor protein which has no cofactor yet (inactive)
How does the apoenzyme turn into a holoenzyme
Cofactor is added (enzyme is activated)
To active the enzyme there must be a change in tertiary structure
How can an enzyme be activated (change in tertiary structure)?
Provide an example for this
-Action of another enzyme
-Change in conditions such as pH or temperature
-Cofactors
Example: inactive pepsinogen released into stomach to digest proteins, acid pH changes pepsinogen into active enzyme pepsin
What are cofactors? Provide an example
Nonprotein inorganic component of an enzymes, some enzymes need these to assist in carrying out their function
Ions are cofactors as they are inorganic
Example: chloride ions are cofactor for amylase
How are cofactors obtained?
Via the diet as minerals
What are coenzymes? Provide an example
Organic cofactors derived from vitamins.
Example: NAD and NADP coenzymes responsible for the transfer of hydrogen atoms in respiration (NAD) and photosynthesis (NADP)
Similarity between cofactors and coenzymes
Both temporarily bound to the enzyme
What are prosthetic groups? Provide examples
Cofactors which are tightly bound to an enzyme, a permanent feature of enzyme
Example 1: Fe2+ in haemoglobin
Example 2: Zn2+ in carbonic anhydrase for the metabolism of CO2
What are precursor enzymes?
Enzymes that are produced in an inactive form, as some actions need to be controlled and only activated in certain conditions, otherwise damage can be caused
What are inhibitors?
Inhibitors are molecules that prevent enzymes from carrying out their normal functions as catalysts or slow them down.
Process of competitive inhibition
Competitive inhibitors have a similar shape to the substrate of an enzyme, can fit into the active site as well.
This blocks the substrate from entering the active site, enzyme cannot function (inhibited)
Most competitive inhibitors only bind TEMPORARILY, effect is reversible
How does a competitive inhibitor reduce the rate of a reaction?
By reducing the number of substrate molecules binding to their active sites in a given time.
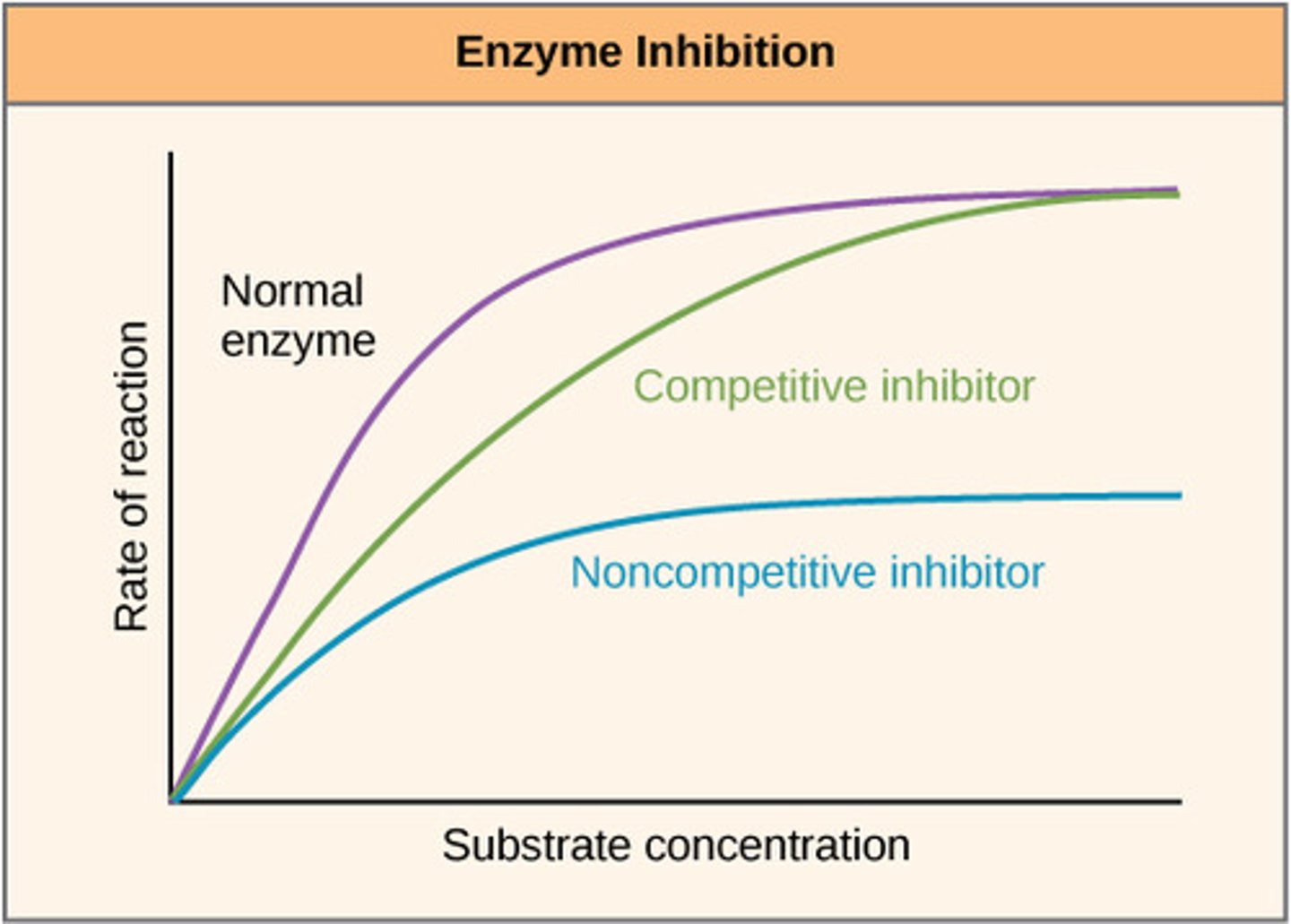
Explain what is happening in the graph for the green line (competitive inhibitor)
-Rate decreases, less available active sites since they have been blocked by competitive inhibitors less enzyme-substrate complexes
-Same V-max reached but at a higher substrate concentration, presence of more substrates overcomes the effect of the inhibitors
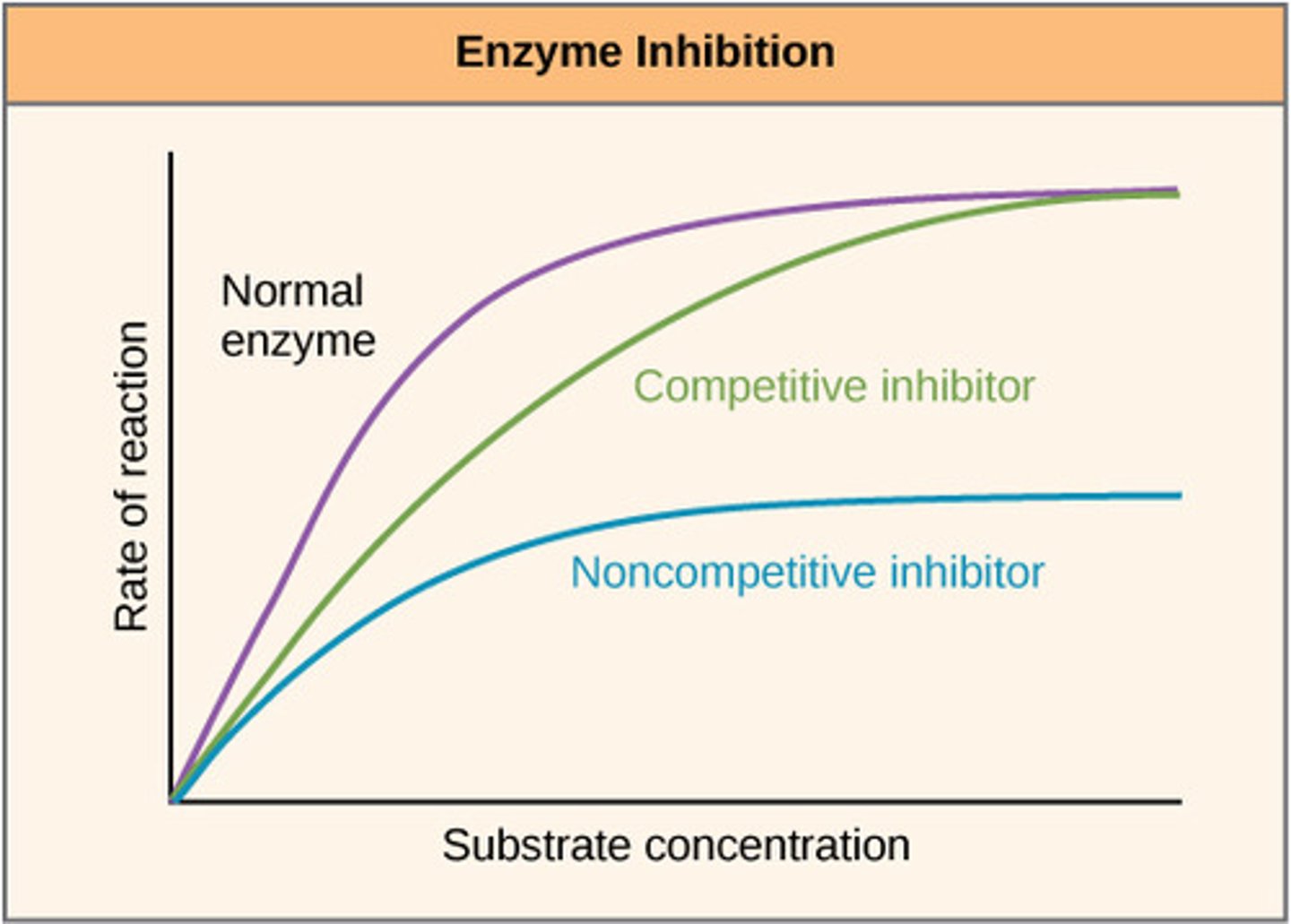
Process of non-competitive inhibition
Inhibitor binds to the enzyme at a location other than the active site (allosteric site).
The binding of the inhibitor results in a change of tertiary structure, so the active site also changes.
The active site is no longer complimentary to the substrate, enzyme cannot carry out tis function (inhibited)
How does a non-competitive inhibitor effect the rate of reaction?
Increasing substrate concentration will not overcome the effect of the inhibitor
Increasing the concentration of the inhibitor reduces the rate of reaction
Explain what is happening in the graph for the blue line (non-competitive inhibitor)
Increasing the substrate concentration does not overcome the effect of the inhibitor as it has caused a permanent change to the tertiary structure, less enzyme substrate complexes
Example of non-competitive irreversible PPI inhibitor
Protein pump inhibitors (PPIs), treat long term indigestion by blocking the enzyme responsible for secreting H ions into stomach, reducing excess stomach acid (no stomach ulcers)
Example of competitive malonate inhibitor
Enzyme sucrose dehydrogenase is involved in respiration, substrate succinate has competitive inhibitor malonate.
This reduces level of respiration
What is meant by end-product inhibition? Explain the process of end-product inhibition
Term used when the product of a reaction acts as an inhibitor for the enzyme that produces it
As the enzyme converts the substrate into products, the process itself is slowed down as the end-product of the reaction binds to an alternative site on the original enzyme, changing the shape and preventing further enzyme-substrate complexes.
The end-product can then detach, active site returns to an active state
How does end-product inhibition work as a negative feedback cycle?
When the end-product binds to the enzyme, it stops the metabolic pathway and prevents further synthesis until the end-product concentration decreases. End product can then detach, active site reforms
How does ATP act as an end-product inhibitor (non competitive inhibition)
-When ATP levels are high, ATP binds to PFK and slows down glycosis which means less ATP is made
-When ATP levels drop (used up), less ATP binds to PFK so glycolysis speeds and makes more ATP
Negative feedback cycle example
Rate of reaction equation
1/time
Graph representing the energy changes involved in an enzyme controlled anabolic reaction
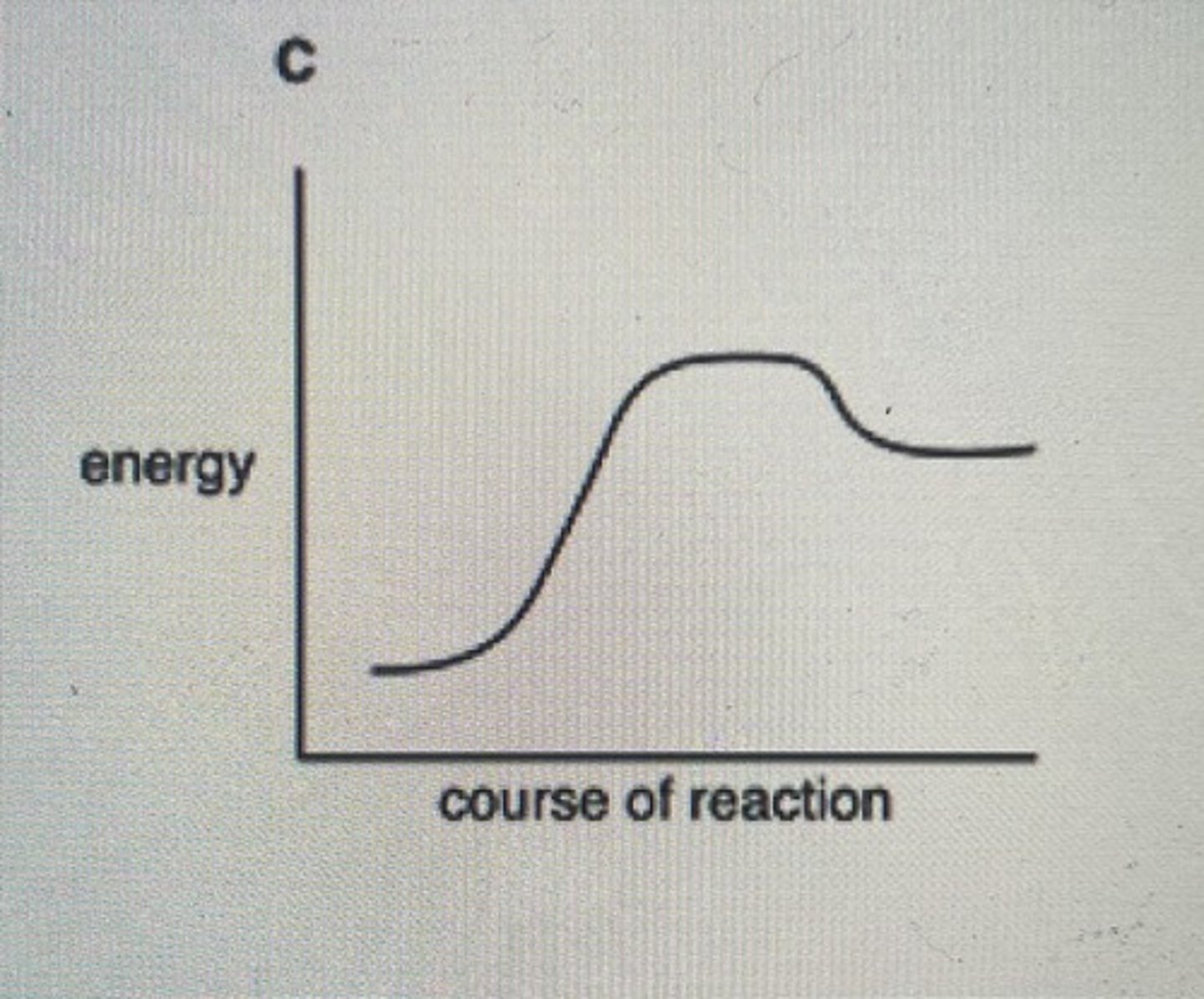
Using the information in the graph, work out the temperature coefficient for the reaction between 20 and 30 degrees Celsius
Rate at 30/rate at 20
20/10 = 2
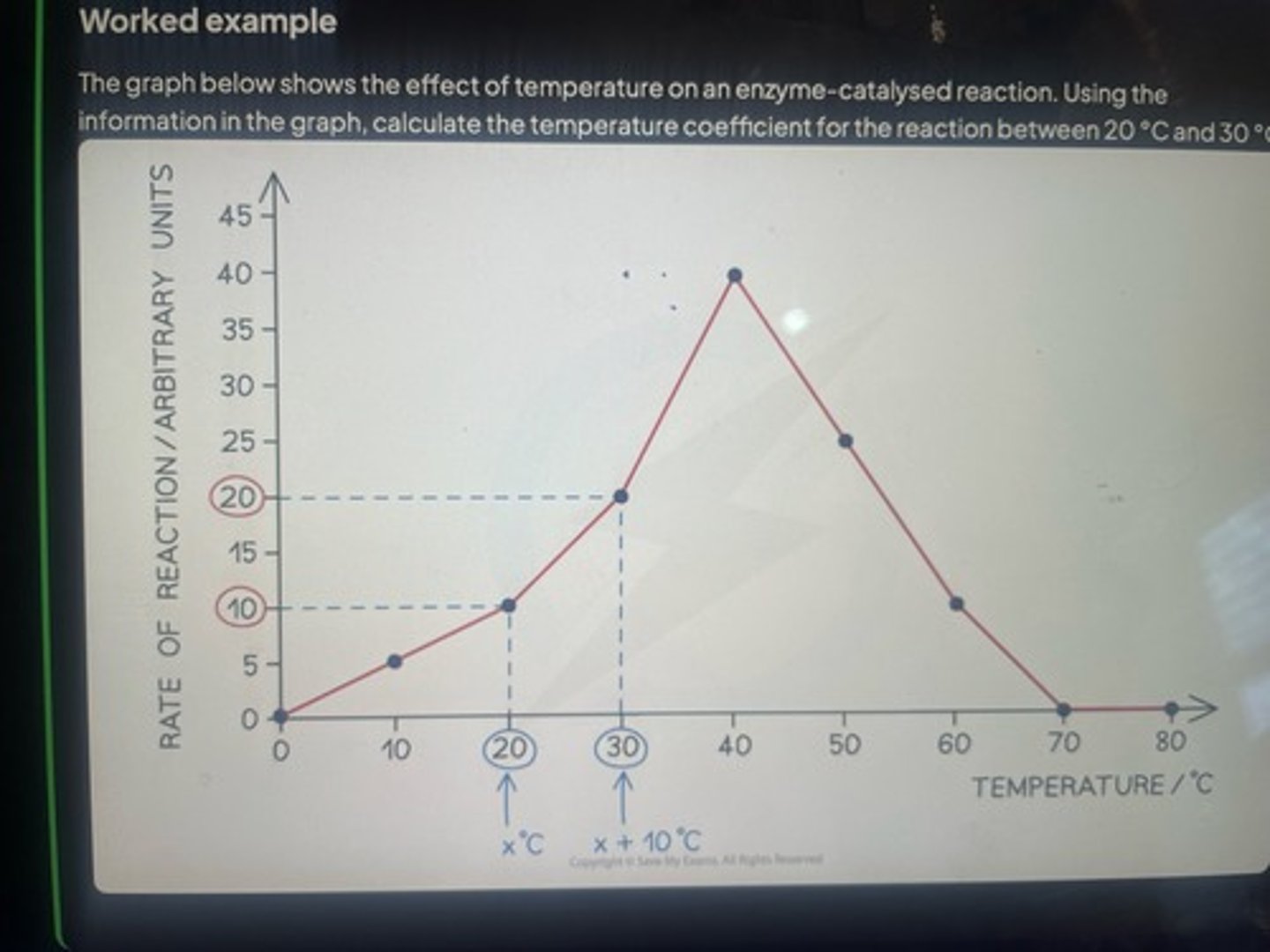
Tissue traces from a crime scene often need to be identified. DNA from the tissue is 'amplified' by the polymerase chain reaction (PCR) to get samples large enough for further analysis .
Modern PCR techniques use DNA polymerase from the bacterium Thermus aquaticus. Why was this enzyme chosen?
Thermostable/does not denature at 95 degrees
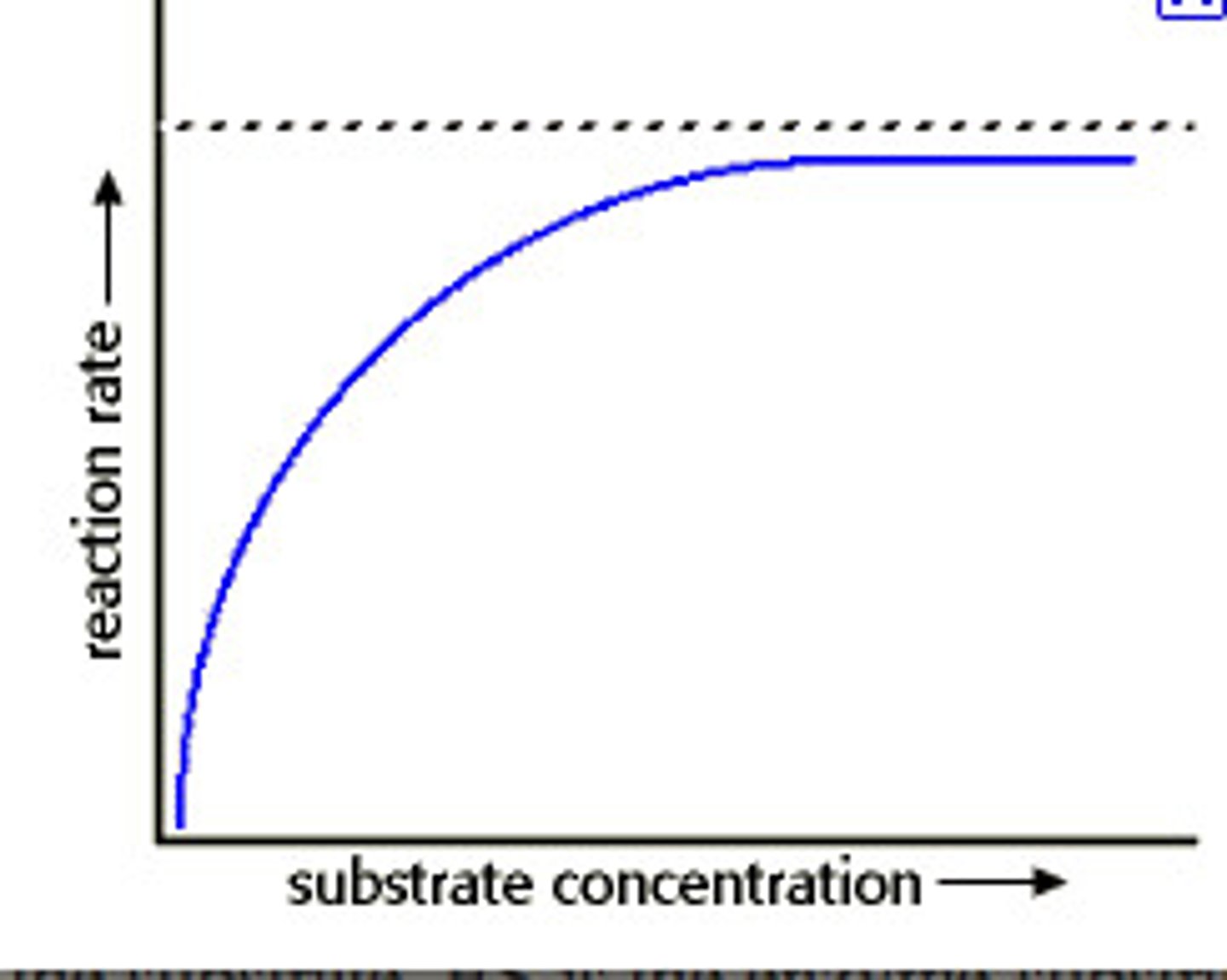
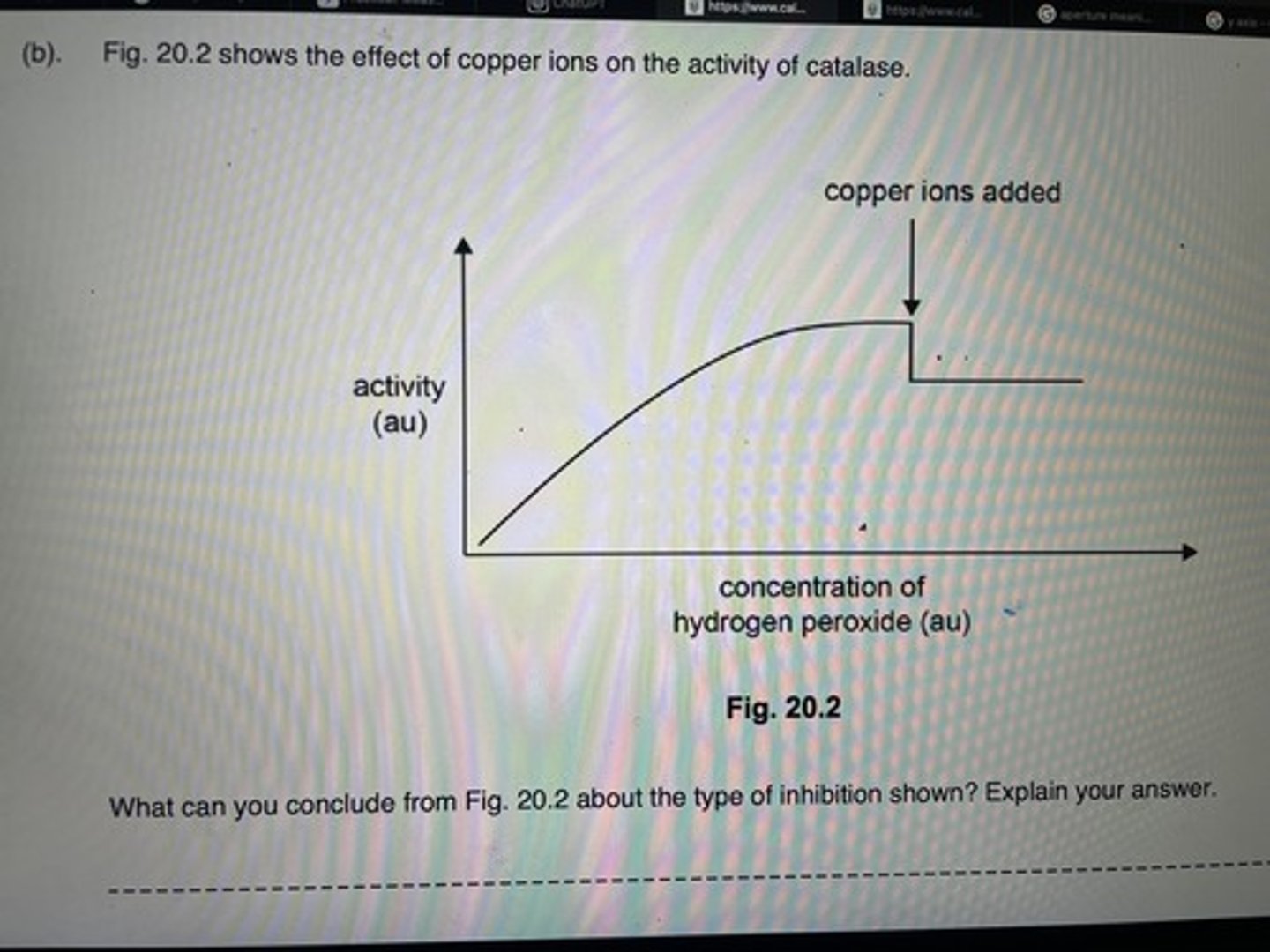
Graph showing effect of substrate concentration on an enzyme controlled reaction
What can you conclude about the type of inhibition show from the graph? (3)
Non competitive inhibition/rate of reaction does not continue to rise as substrate concentration does/this would be so in competitive inhibition
Some enzymes work better in the presence of other molecules or ions. Explain how these molecules or ions increase the activity of enzymes (5)
Cofactors/bind to the enzyme temporarily/ this changes the shape of the active site/ there is an increased likelihood of a successful collision/ more ESC formed/ carry chemicals between enzymes
Chitinase is an enzyme produced by plants which catalyses the breakdown of chitin in the cell walls of fungi
i) two amino acids in chitin's move closer together to form a hydrophobic region around the chitin substrate
ii) other amino acids interactions cause the active site of the enzyme to partially cover the chitin substrate
A) name the hypothesis of enzyme action in this mechanism
b) explain how the mechanism of enzyme action observed in chitinase increases the rate of chitin breakdown
a) induced fit, chitinase forms a hydrophobic region around the chitin substrate (changing the shape slightly)
b) change in active site causes formation of stronger bonds between chitin and chitinase. Bonds in chitin are weakened (put under strain), lowering the activation energy