4.1 & 4.2 Enzymes and Factors Affecting Enzyme Activity
1/56
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
Fill in the blanks: Enzymes are (b)____ (they function in ____ systems) (c)____ (they ___ the rate of chemical reaction without being ____ or undergoing ____ change)
biological, living, catalysts, increase, used up, permanent
What type of proteins are enzymes and is their tertiary structure complex or simple?
globular proteins with a complex structure
True or false? all anabolic (building up) and catabolic (breaking down) reactions are catalysed by enzymes
true
What is the metabolism of an organism?
the sum of all the different reactions and reaction pathways happening
What are the factors affecting enzyme activity?
temperature, pH, concentration of substrates and enzymes
What is the Vmax of an enzyme?
The maximum initial velocity or rate of the enzyme-catalysed reaction
What are intracellular enzymes?
enzymes that are produced and function inside the cell
What are extracellular enzymes?
enzymes that are secreted by cells to catalyse reaction outside of cells
Give an example of an intracellular enzyme and its function
e.g. catalase - found in hepatocytes (liver cells) that detoxifies toxic hydrogen peroxide to water and oxygen
Give an example of an extracellular enzyme and its function
e.g. digestive enzymes (amylase, pepsin, trypsin and lipase) are secreted into the digestive tract as the macromolecules being digested are too large to enter the cell
How do enzymes speed up the rate of reactions?
they reduce the activation energy
What is the lock and key hypothesis?
the active site of an enzyme has a specific and complementary 3D shape to the substrate
a complementary substrate successfully collides with an binds to the active site from in an enzyme-substrate complex (ESC)
the enzymes catalyses the reaction by lowering the activation energy
an enzyme-product complex (EPC) is formed
the products of the reaction are released and the enzyme remains unchange
If an enzyme breaks down a substrate in catabolic reaction, what type of reaction is taking place?
hydrolysis
Fill in the blanks: the ___ fit hypothesis suggests that there is an (i)____ (i)____ between the enzyme and the substrate. There is a slight ____ shape change in the ____ when forming the ____. This interaction is ____, but it rapidly induces changes in the enzyme’s ____ structure, which weakens the ___ so that more/less _____ ___ is now needed to break the bonds.
induced, initial interaction, conformational, enzyme, ESC, weak, tertiary, bonds, less, activation energy
Why is the product released from the EPC in the induced fit hypothesis?
the product no longer fits into the active site so moves away, allowing the enzyme to be reused
In an anabolic reaction, how does the conformational shape change of the enzyme make it easier for the bonds to form between the two substrates?
the enzyme pushes the two substrates closer together so it is easier for the bonds to form over a shorter distance
Explain in detail how increasing temperature increases the rate of reaction in an enzyme controlled denaturation (up to the optimum)
increasing temperature increases the kinetic energy of the particles, which are in Brownian motion, causing them to collide more frequently with a greater force
therefore there are a greater number of collisions and successful collisions in a given time
this means that more ESC and EPC are formed in a given time increasing the rate of reaction
Fill in the blanks: at the optimum temperature, enzyme activity is at the ___, meaning there are the ____ number of (s)____ (c)____
Vmax, maximum, successful, collisions
What happens to enzyme activity at low temperatures?
there is very little kinetic energy, so particles move slowly in Brownian motion, leading to there being less collisions and successful collisions, so less ESC and EPC are formed
What does ESC stand for?
enzyme-substrate complex
What does EPC stand for?
enzyme-product complex
What biological feature of enzymes causes them to be affected by temperature?
they are proteins
Fill in the blanks: in enzyme denaturation, the temperature (and ___ ___) increase so much that the ___ of the enzyme’s molecules (s)____ the bonds until they ____, which causes the active site to lose it’s ___ ____ shape so that it is no longer ___ to the substrate.
kinetic energy, vibration, strain, break, 3D, conformational, complementary
True or false? the denaturation of enzymes due to temperature is irreversible
true
Which bonds found in the tertiary structure of an enzyme are broken and in what order?
hydrogen bonds (break first), then ionic and hydrophilic/phobic interactions break
Which bonds in the tertiary structure of an enzyme don’t break in denaturation?
disulphide bridges
What is the optimum temperature of most enzymes?
40-50ºC
Fill in the blanks: ____ are organisms that can live in very hot environments, who have ____ enzymes that can function at over __ºC
thermophiles, thermostable, 80
How can you find the initial rate of reaction on a graph with the rate of reaction on the y-axis and the independent variable on the x-axis?
calculate the gradient of a tangent
Why is the rate of an enzyme-catalysed reaction higher at the start?
there is a higher concentration of substrate molecules
What term can be used instead of “all the active sites are full”
occupied
What is a term describing the fast initial rate of reaction?
burst phase
What is the temperature coefficient?
a measure to of the rate of change of a biological or chemical system as a consequence of increasing temperature by 10ºC. In other words, the sensitivity of enzymes to changes in temperature.
What is the symbol of the temperature coefficient?
Q10
What is another word for the temperature coefficient?
temperature quotient
Does Q10 have units? Why/why not?
no - it is a ratio between the rate of reactions at different temperatures
What is the temperature coefficient of a reaction that isn’t temperature sensitive?
Q10=1
Describe what Q10=2 means for a reaction
the rate of reaction doubles for every 10ºC rise in temperature
What are most biological Q10 values between?
2-3
What is the formula for calculating the temperature coefficient of a reaction?
Q10=Rt / Rt-10 (rate at temperature/rate at temperature 10ºC lower)
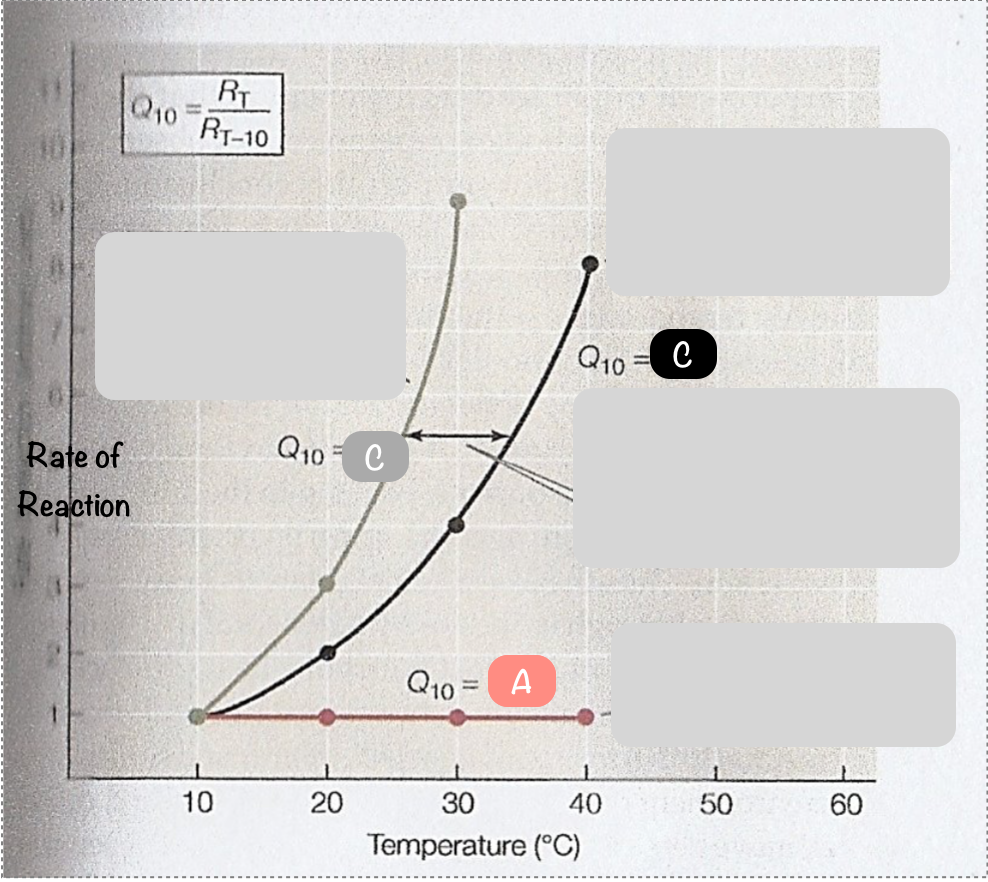
What would be the temperature coefficient for the line A?
1
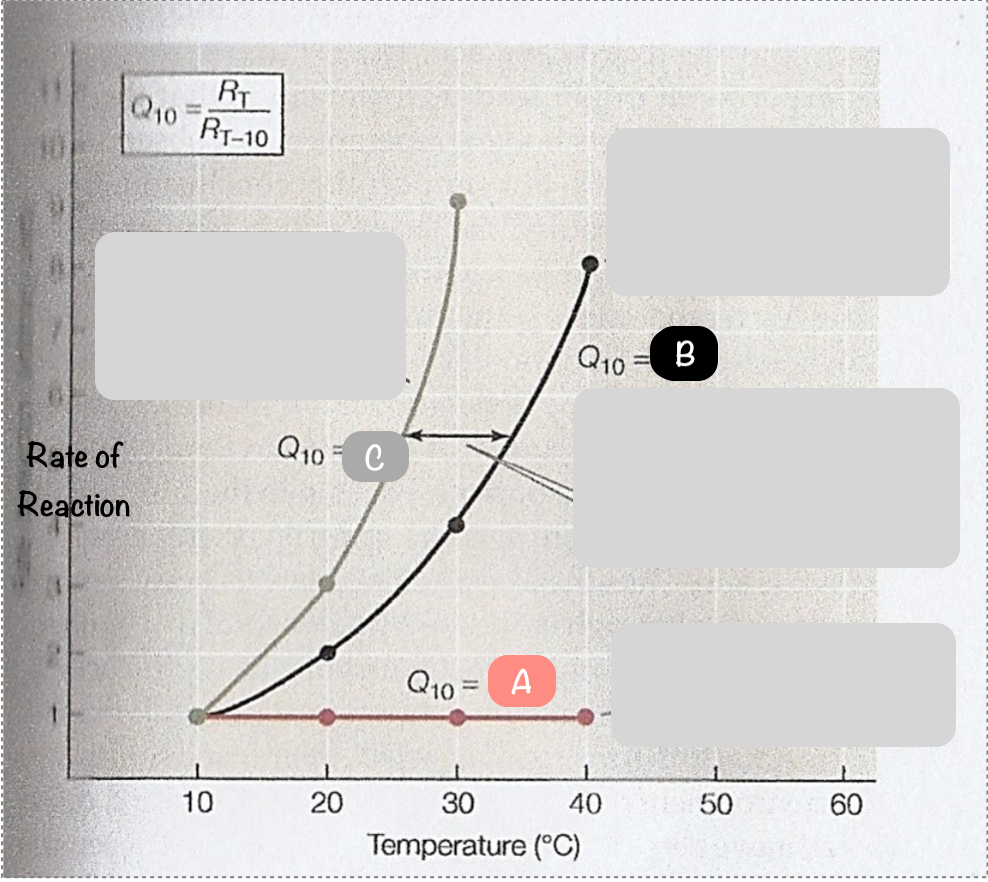
Which line, A, B or C, would have the highest temperature coefficient?
C
What is pH a measure of?
hydrogen (H+) ion concentration
How can hydrogen ions in an acidic solution affect an enzyme?
they distrust the ionic interactions and hydrogen bonds in the enzyme’s tertiary structure
How can hydrogen ions in an acidic solution affect the ionic interactions in an enzyme’s tertiary structure?
they can be attracted to the negatively charged R-groups around the active site, where they ‘cluster’ together, which interferes with the binding of the substrate to the active site
How can hydrogen ions in an acidic solution affect the hydrogen bonds in an enzyme’s tertiary structure?
the hydrogen bonds within the tertiary structure can bond with the H+ ions around the amino acids in an acidic solution, which changes the shape of the active site, preventing the interaction of R-gourps to maintain the 3D shape
Describe what happens to an enzyme’s activity at the optimum pH
there are an ideal number of H+ ions in the solution, so the ionic bonding if the enzyme isn’t disrupted and so the shape of the active site is unchanged. This makes it specific and complementary to the substrate, so ESC and EPC can be formed at the maximum rate (Vmax)
True or false? changes to an enzymes tertiary structure due to pH can be reversible
true
What is the term used to describe the reversible changes in enzyme structure due to pH?
renaturation
Explain what happens to an enzyme when there is a slight change to pH either side of the optimum
renaturation occurs as the tertiary structure of the enzyme (and the shape of the active site) is only slightly altered so that if the pH returns to the optimum the protein will resume its normal shape and catalyse the reaction at the Vmax again
Explain what happens to enzymes in denaturation due to pH
a large increase or decrease in the concentration of H+ ions disrupts the ionic bonding, changing the tertiary structure of the enzyme and the 3D shape of the active site so it is no longer specific and complementary to the substrate meaning that no ESC and EPC can form
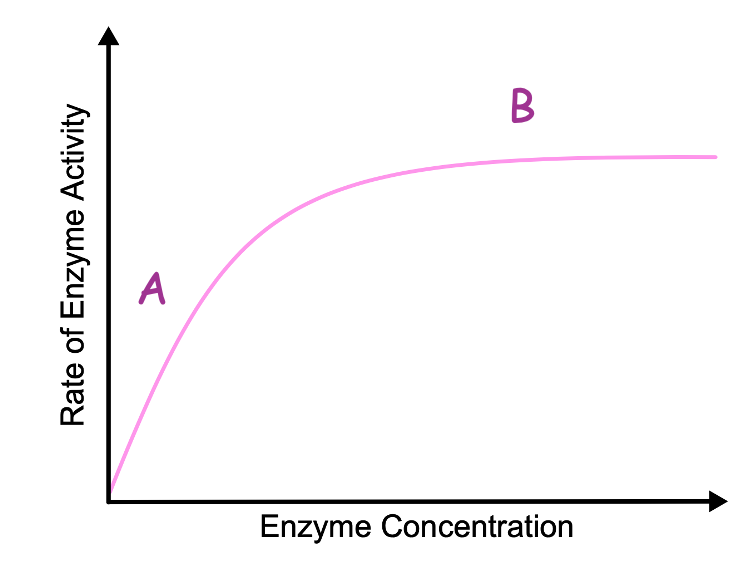
The graph shows the effect of enzyme concentration on the rate of enzyme activity. Describe what is happening at A.
The rate of reaction increases linearly with enzyme concentration

The graph shows the effect of enzyme concentration on the rate of enzyme activity. Describe and explain what is happening at B
The Vmax of the reaction is reached as all the active sites of the enzymes are occupied by substrate particles so no more ESC’s can be formed until the products are released

The graph shows the effect of enzyme concentration on the rate of enzyme activity. What is the limiting factor at point B
substrate concentration
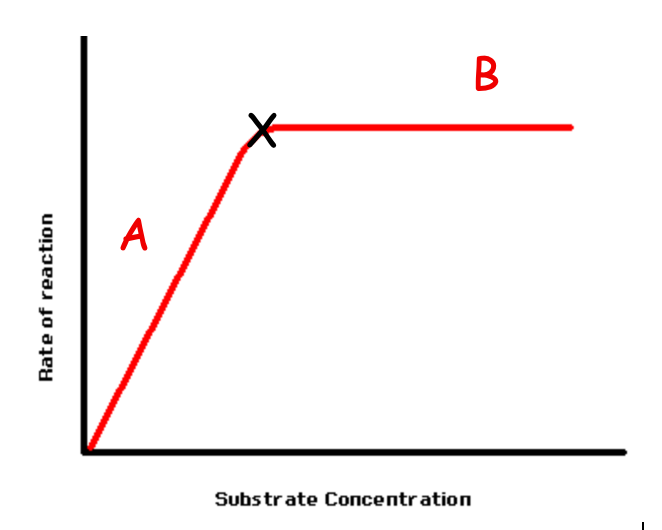
The graph shows the effect of substrate concentration on the rate of enzyme activity. Describe and explain what is happening at A
The rate of reaction increases linearly with substrate concentration as there is an increasing rate of collisions with the active sites of enzymes forming more ESC and EPC

The graph shows the effect of substrate concentration on the rate of enzyme activity. Describe and explain what is happening at B
The rate of reaction has plateaued as the reaction has reached the Vmax with all the active sites of the enzymes occupied, meaning that no more ESC can form. The reaction has become saturated

The graph shows the effect of substrate concentration on the rate of enzyme activity. What is the name given to the point X
point of saturation