CHEM 2300 / Topic 2a: Lewis Acids and Bases - From Kelly
1/21
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Lewis acid
Electron-pair acceptor, having an empty orbital or ability to rearrange electrons to create an empty orbital, forming new bonds.
Lewis base
Electron-pair donor, having an accessible lone pair of electrons to donate to form a new bond.
Adduct
The product of a reaction between a LA and LB, held together by a newly formed covalent bond.
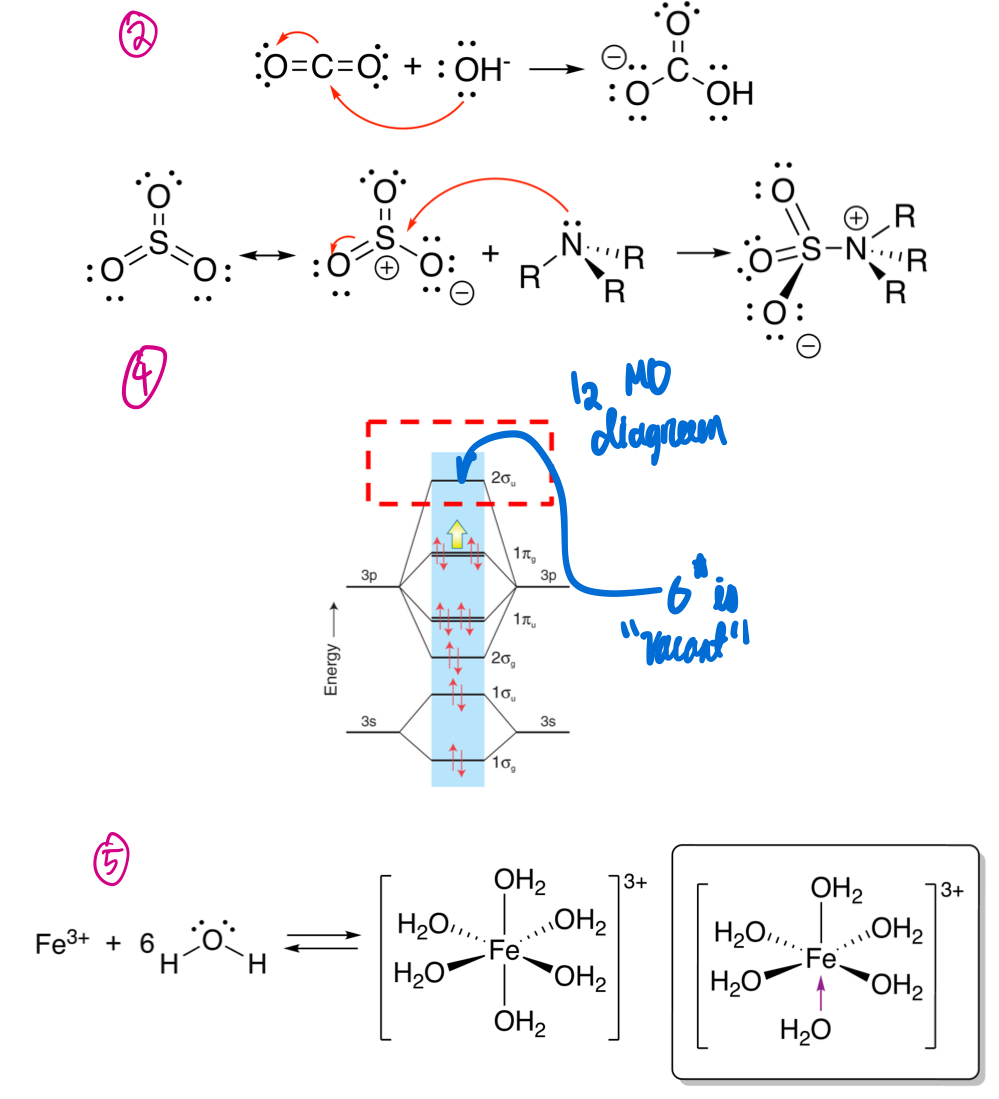
How can LAs and LBs relate to MO theory?
The LUMO is the empty orbital of a Lewis acid, while the HOMO is the filled orbital of a Lewis base.
In the perspective of MO, how do adducts form, where do they get their stability from?
Via overlap of the LUMO and the HOMO, the adduct is stabilized by a net lowering of energy of these orbitals together.
In the perspective of MO, what can the adduct be viewed as?
The adduct can be viewed as a new pair of MOs - a BMO and ABMO.
How does energy of each of the HOMO and LUMO relate to the strength of Lewis acidity and basicity, and why?
The lower the energy of the LUMO, the stronger the Lewis acid.
The higher the energy of the HOMO, the stronger the Lewis base.
Lewis-base adduct
A compound formed by the interaction between a Lewis base and a Lewis acid, where the Lewis base donates a pair of electrons to the Lewis acid.
Coordinate bond
Also known as a dative bond, this is a type of covalent bond wherein both shared electrons come from the same atom.
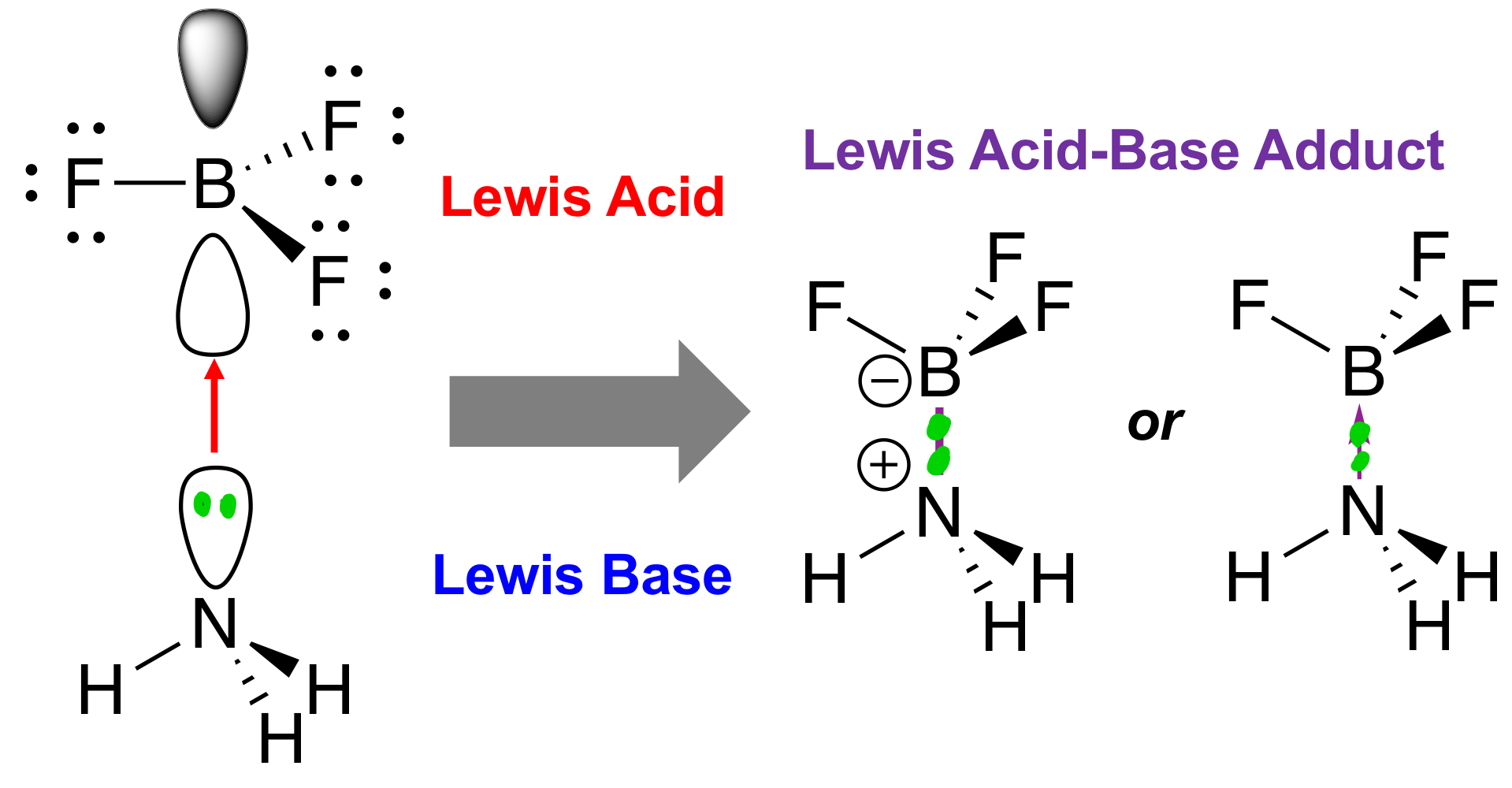
How do electrophiles and nucleophiles relate to Lewis acids and Lewis bases? What’s to note about their usage in explaining reactions?
Electrophiles are Lewis acids, while nucleophiles are Lewis bases. Electrophiles and nucleophiles usually talk about kinetics, while Lewis acids and Lewis bases usually talk about equilibrium properties of reactions. In a sense, it talks about the thermodynamics and favourability of reactions.
What are the types of Lewis acids?
Molecules with incomplete octets of valence electrons
Molecules with complete octets can rearrange their valence electrons to accept an electron pair, e.g. via resonance.
Atoms on molecules being large enough to accept another electron pair.
Group 17 diatomics, which are mildly acidic but has a vacant ABMO regardless.
Metal cations, which can accept Lewis bases, like solvents, to form coordination complexes.
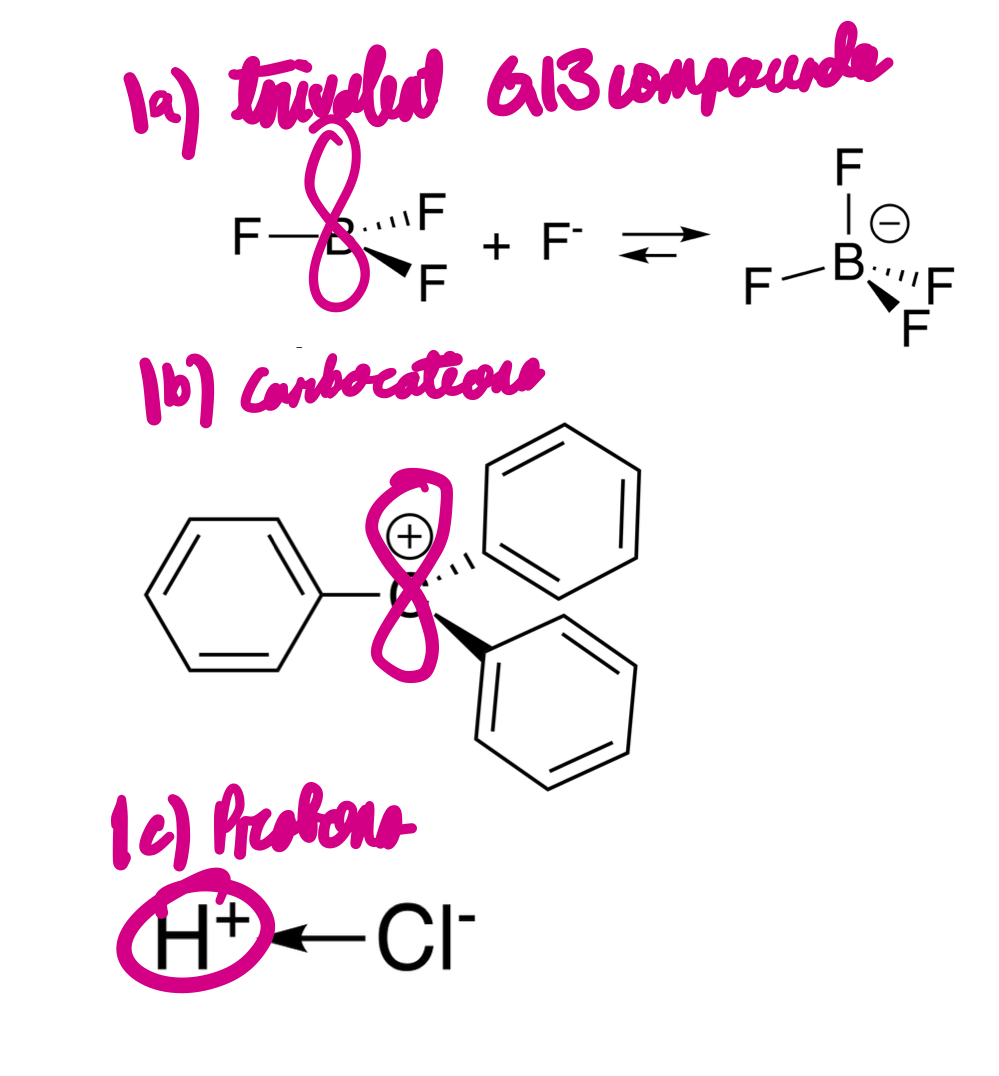
There are three types of molecules with incomplete octets of valence electrons, acting as Lewis acids. What are these three types?
Trivalent group 13 compounds, carbocations, and protons.
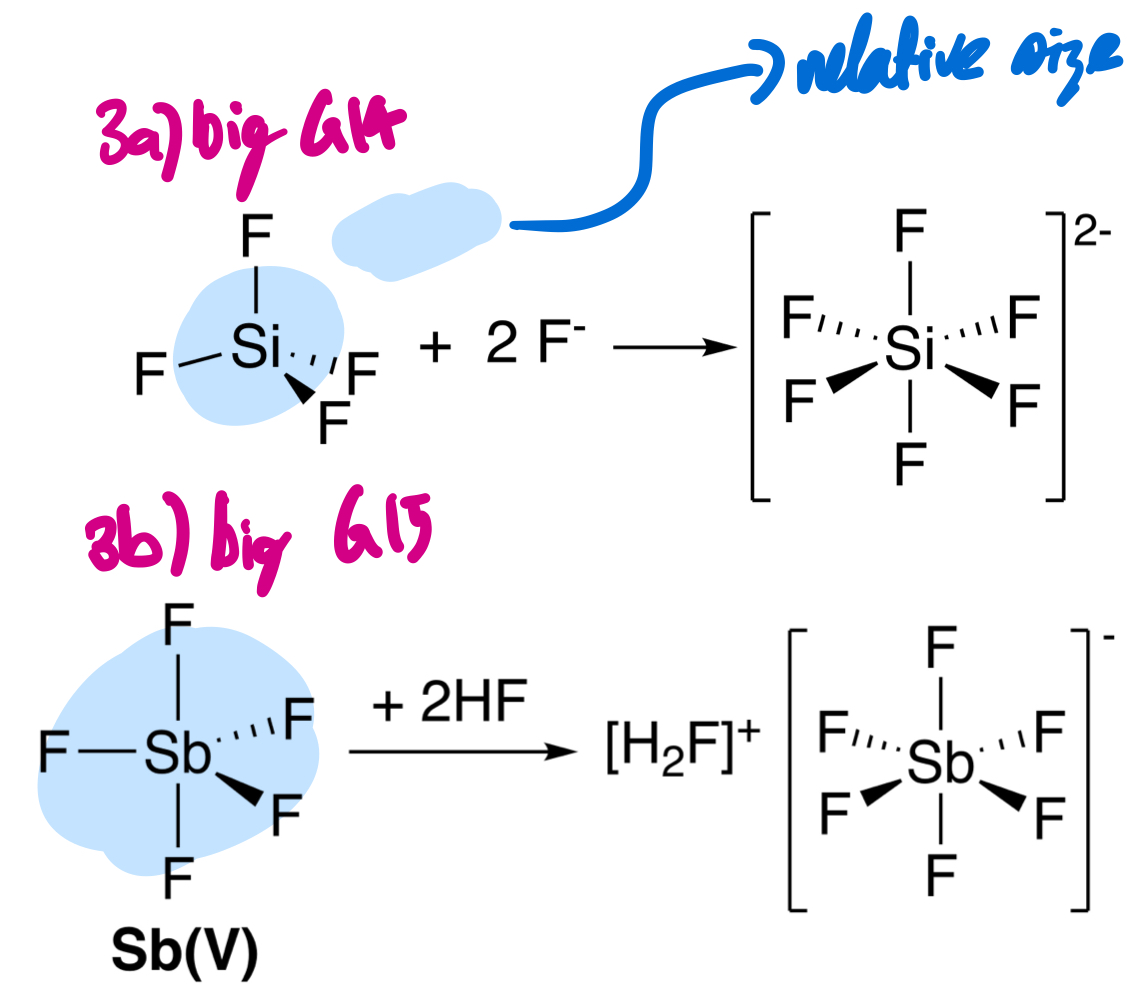
There are two types of atoms on molecules being large enough to accept another electron pair, acting as Lewis acids. What are these two types?
(1) Group 14 compounds with tendency to act as acids by becoming 5- or 6-coordinate, and (2) Group 15 heavy pentahalides.
What are the five types of Lewis bases?
Halide anions, e.g. Br-, I–, etc.
Hydroxide and hydride anions, i.e. OH-, H-.
Xenon.
Neutral molecules with accessible lone pairs.
Neutral molecules with electrons in bonding orbitals.
There are three types of neutral molecules with accessible lone pairs that can act as a Lewis base. What are these three types?
Trivalent group 15 compounds: amines (R3N), phosphines (R3P), arsines (R3As), stibines (R3Sb).
Donor solvents: H2O and CH3CN.
Divalent group 14 compounds: carbenes (R2C), stannylenes (R2Sn), and plumbylenes (R2Pb).
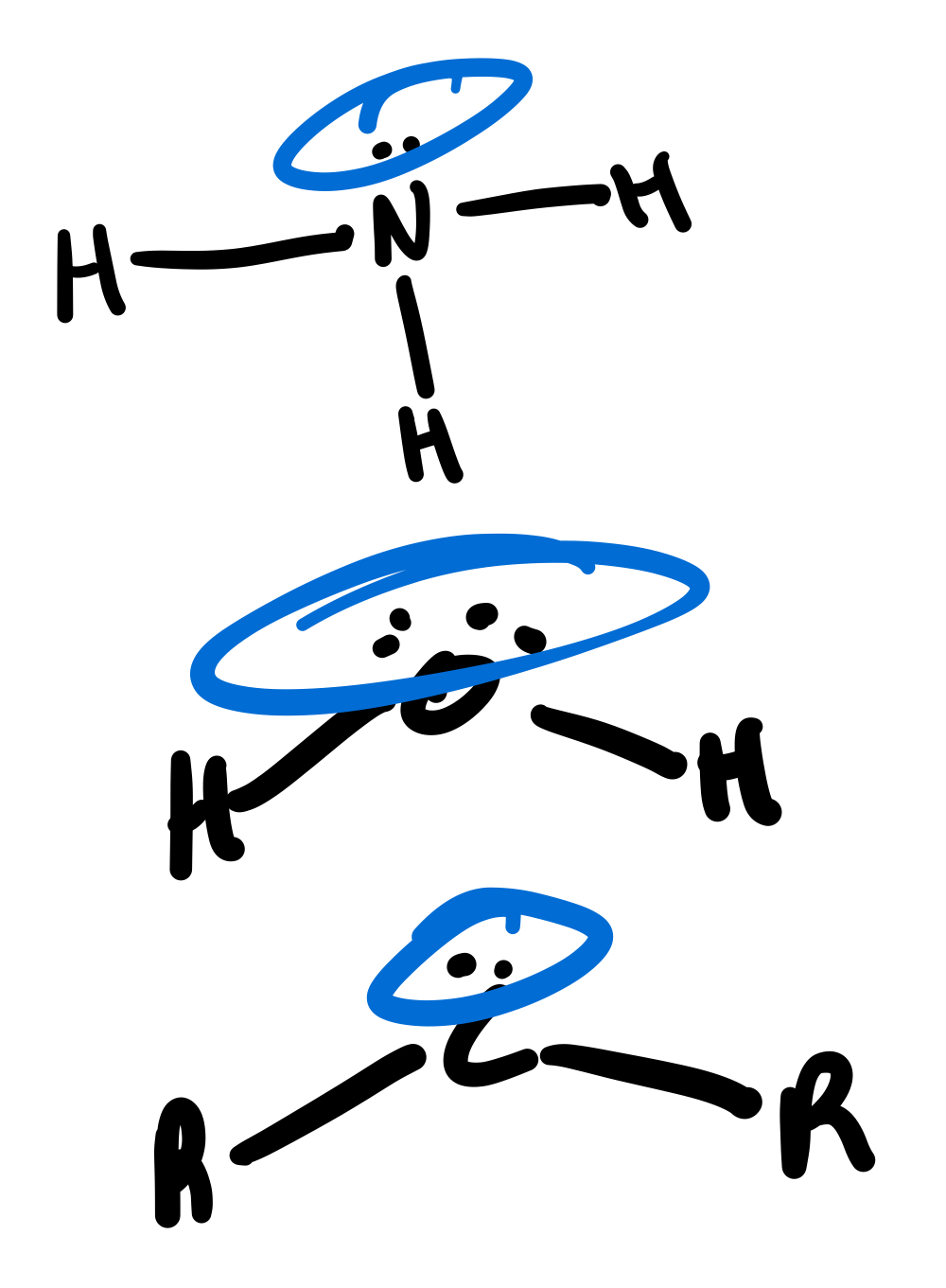
There are two types of neutral molecules with electrons in bonding orbitals that can act as a Lewis base. What are these two types?
π-bonding pair of electrons, such as in ethene.
σ-bonding pair of electrons, such as in B2H6.
Formation constant (Kf)
The constant associated with adduct formation, measuring strength of Lewis acid-base interaction.
When measuring strengths, what does the strength of the Lewis acid depend on, and what does the strength of the Lewis base depend on? Why is this so?
The strength of the Lewis acid will depend on the strength of a Lewis base, and vice versa. There is no universal scale. There is only the relative scale.
When
Steric effects don’t affect the strength of the Lewis acid when the most important atom becomes bigger.
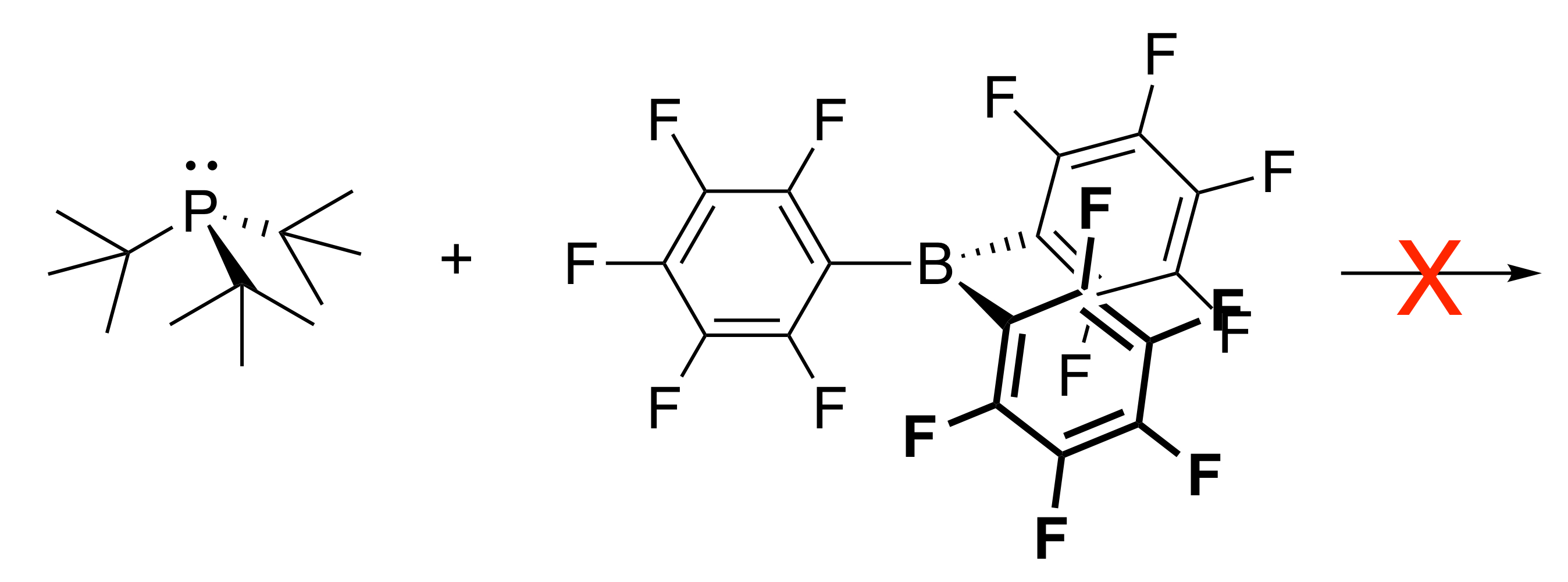
What’s to note regarding steric effects when trying to make an adduct?
If you have Lewis species with bulky groups on both, or having bulky groups to begin with, adducts will not be able to be formed because the steric interactions need to find a way to bond in all the bulky madness.
What does the hard-soft acid-base theory state? For an example, if a hard acid was paired with I- and F- individually, how would this affect the Kf? How about if it was a soft acid?
Hard/hard acid-base or soft/soft acid-base adducts tend to be more stable (i.e. more common and having a large Kf) than mixed combinations.
For a hard acid, Kf is smallest when paired with I- and largest with F-.
For a soft acid, Kf is smallest when paired with F- and largest with I-.
Generally, how does ∆Hrxn relate to the hard-soft acid theory?
Hard/hard interactions will be more ionic in bonding character. Soft/soft interactions will be more covalent. If these interactions occurred in the same reaction, then there will be a large ∆Hrxn, corresponding to a large decrease in potential energy and thus large increase in stability.