BIOC 303: Cholesterol
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Cholesterol Synthesis: Acetate
cholesterol is a 27 C compound, in which all C atoms are provided by acetate
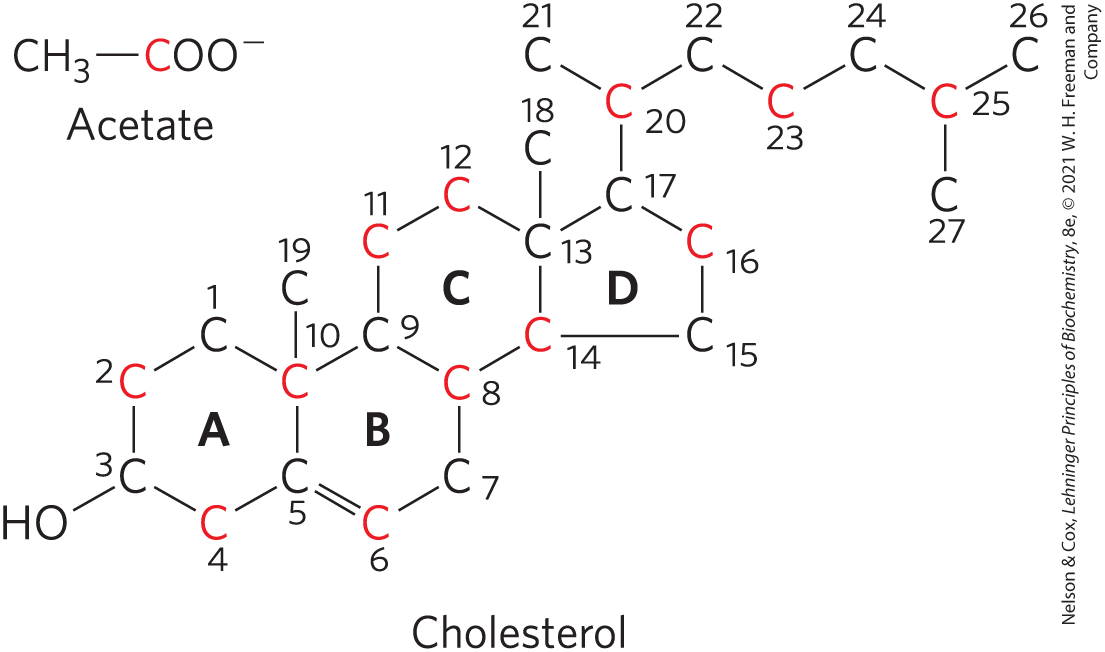
Cholesterol Synthesis: Isoprene
isoprene units are essential intermediates in the pathway
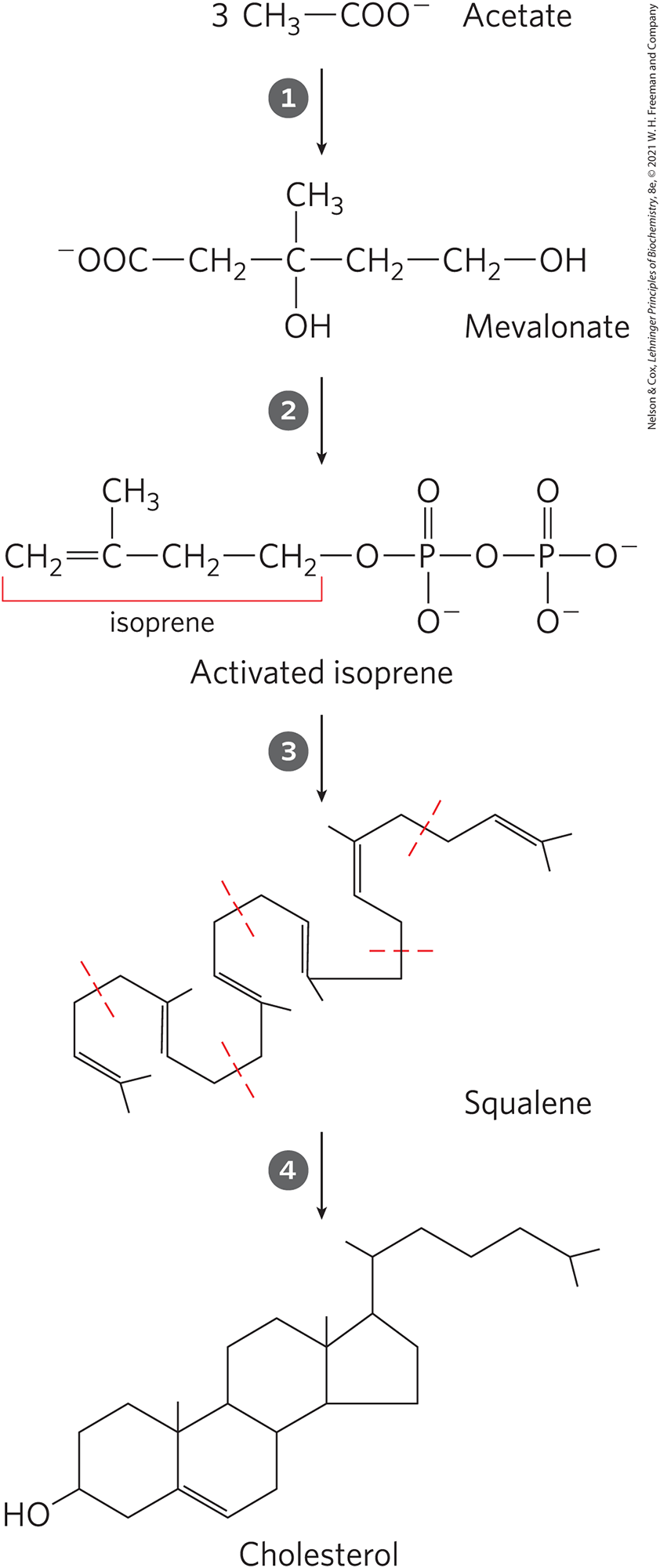
Cholesterol Synthesis Steps
condensation of 3 acetate units to form a six-carbon intermediate, mevalonate
conversion of mevalonate to activated isoprene units
polymerization of six 5-carbon isoprene units to form the 30 carbon liner squalene
cyclization os squalene to form the 4 rings of the steroid nucleus, with a further series of changes (oxidations, removal or migration of methyl groups) to produce cholesterol
Cholesterol Synthesis Figure
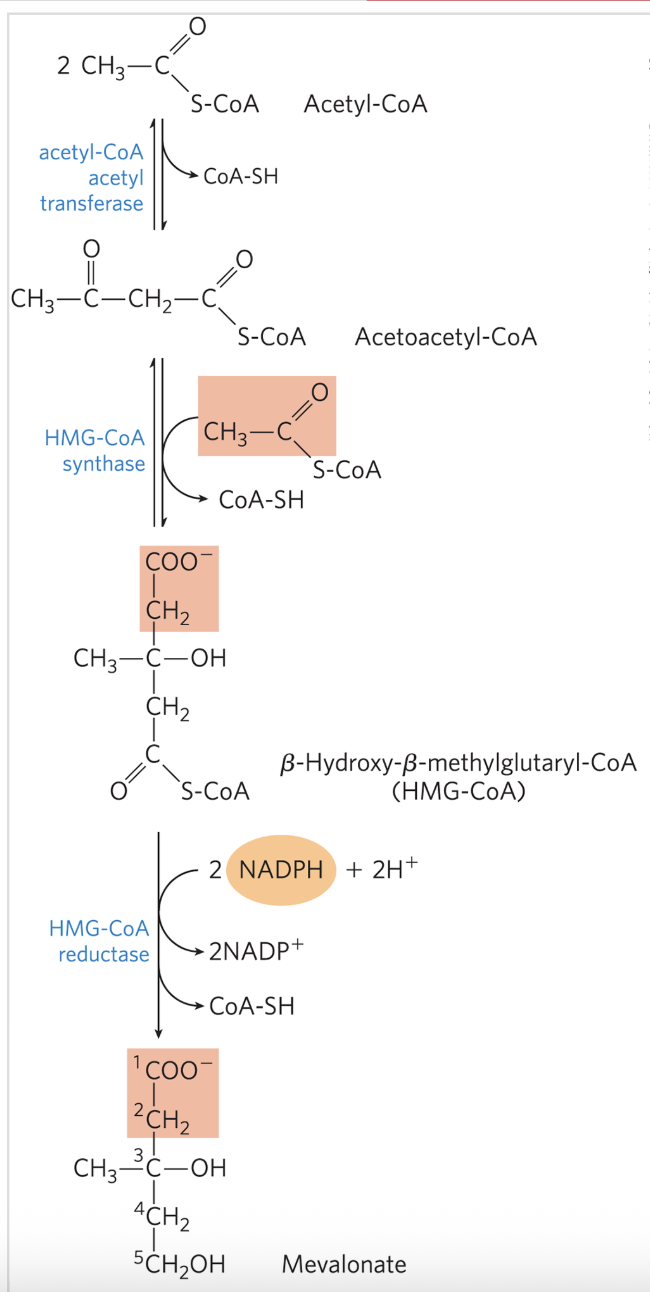
Stage 1: Mevalonate Synthesis from Acetate
two molecules of acetyl-CoA condense to form acetoacetyl-CoA, catalyzed by acetyl-CoA acetyl transferase
HMG-CoA synthase catalyzes the condensation of acetyl-CoA with acetoacetyl-CoA to form β-hydroy-β-methylglutaryl-CoA (HMG CoA)
both are Claisen condensations and the standard equilibrium in both favors degradation to acetyl-CoA
however the HMG-CoA is rapidly used after synthesis in subsequent reactions
reduction of HMG-CoA to mevalonate by HMG-CoA reductase
the committed step; major point of regulation on the pathway to cholesterol
requires 2 NADPH
Stage 1: Mevalonate Synthesis from Acetate FIGURE
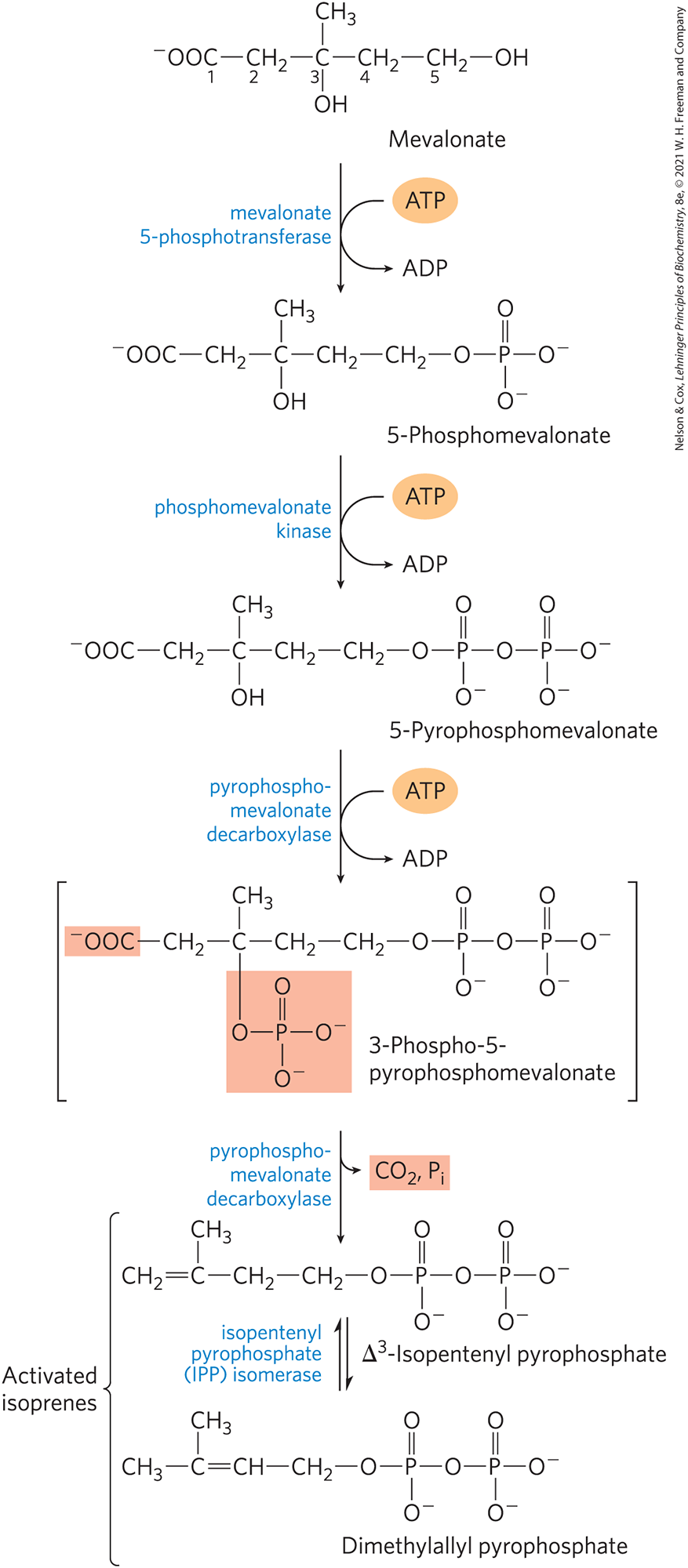
Stage 2: Conversion of Mevalonate to 2 Activates Isoprenes
3 phosphate groups are transferred from 3 ATP molecules to mevalonate to produce the intermediate, 3-phospho-5-pyrophosphomevalonate
3 ATP for each activated isoprene → total of 18 ATP
one of the added phosphates (on the C3 of ⬆ intermediate) makes a good leaving group
it leaves and at the same time, a nearby carboxyl group also leaves, forming a double bond in the molecule, yielding ∆3-isopentyl pyrophosphate (IPP)
first of 2 activates isoprenes
isomerization of IPP yields the second activated isoprene, dimethylallyl pyrophosphate (some are isomerized but not all to create 2 pools)
Stage 2: Conversion of Mevalonate to 2 Activates Isoprenes FIGURE
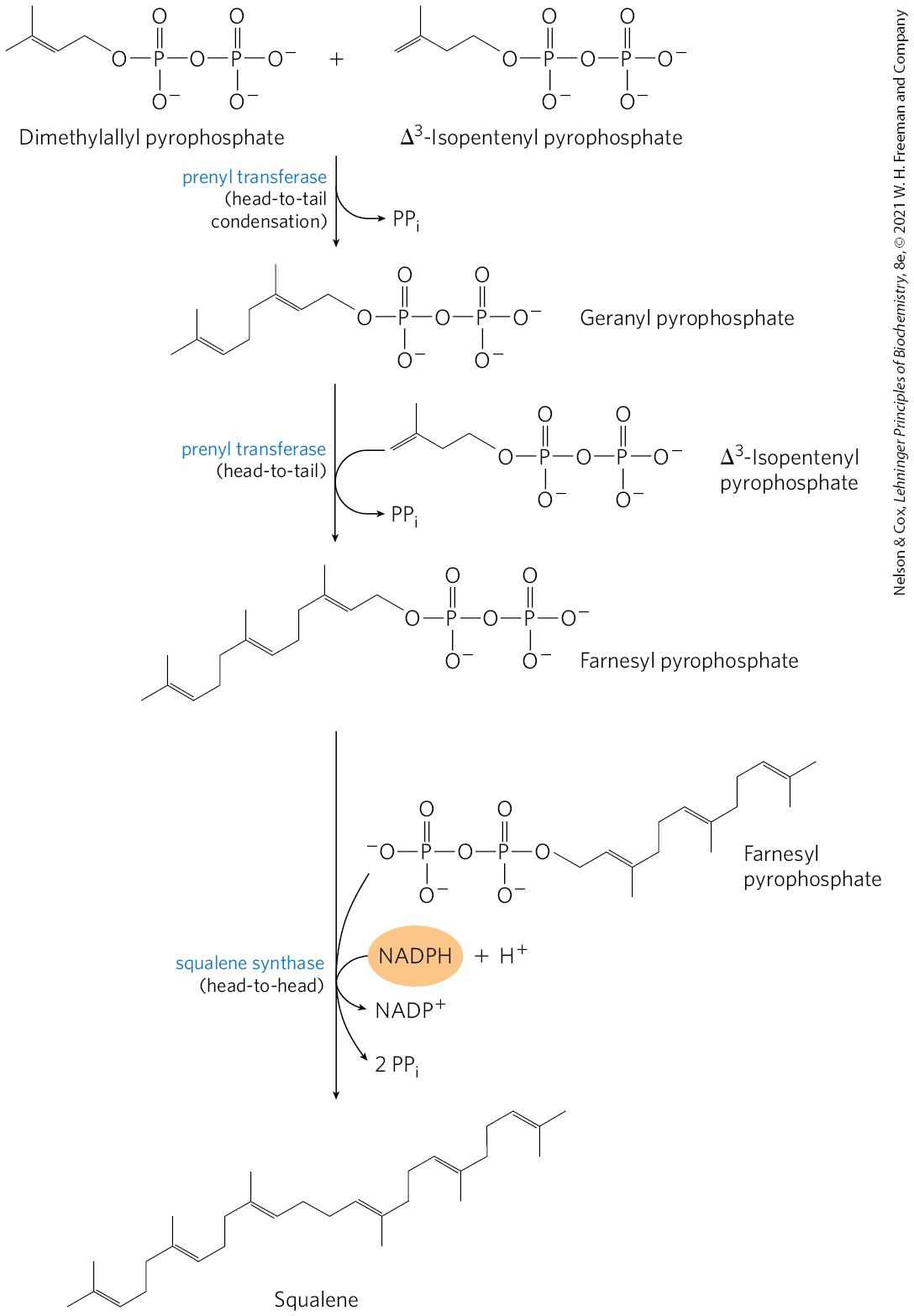
Stage 3: Condensation of 6 Activated Isoprene Units to form Squalene
Isopentyl PP and dimethylallyl PP undergo head-to-tail condensation to form geranyl PP (10 C chain)
one PP group is displaced
geranyl PP undergoes head-to-tail condensation with isopentyl PP to yield farnesyl PP (15 C intermediates)
2 molecules of farnesyl PP join head to head, eliminating both PP groups, to form squalene (30 C’s; 24 in main chain, 6 in methyl group branches)
Stage 3: Condensation of 6 Activated Isoprene Units to form Squalene FIGURE
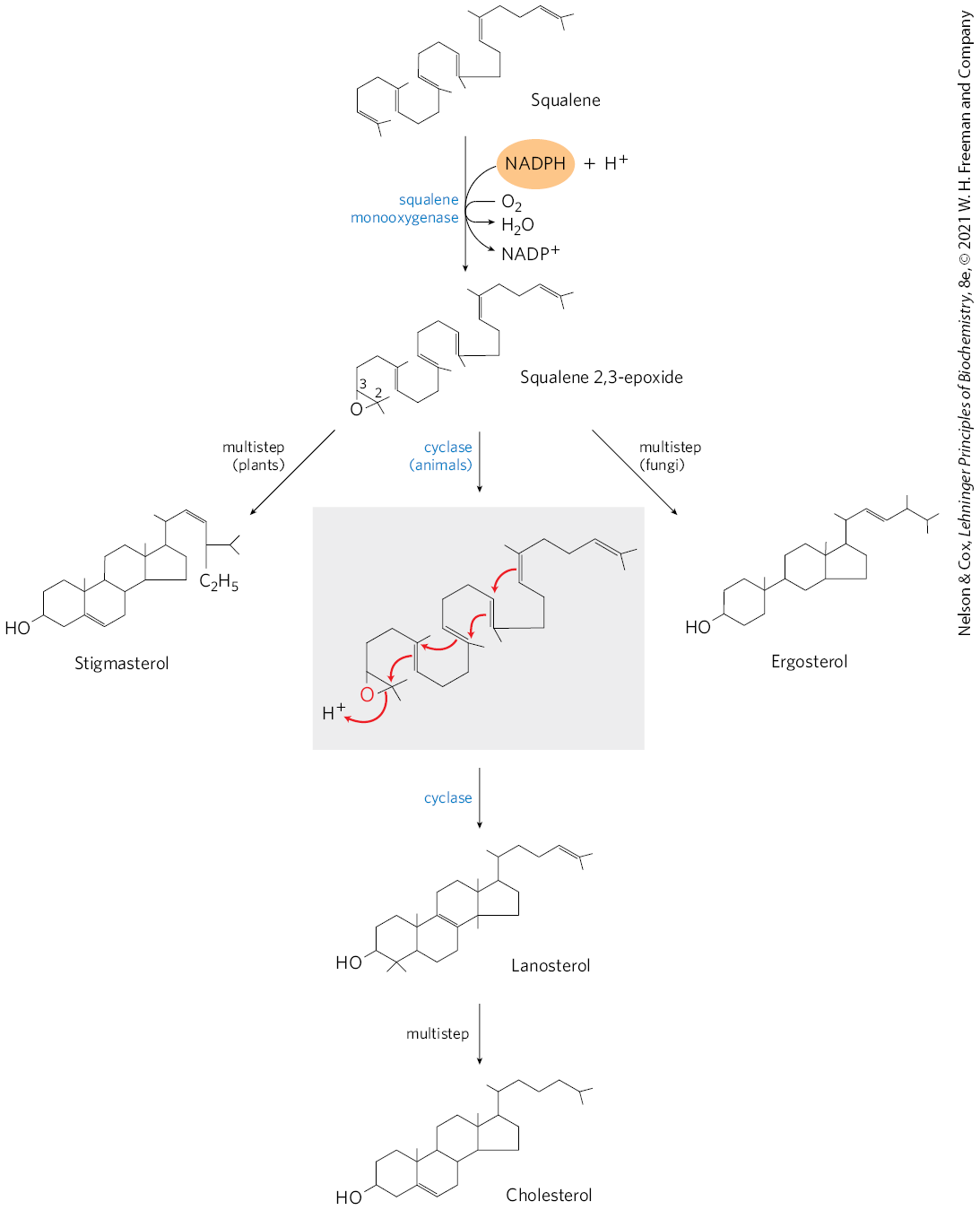
Stage 4: Conversion of Squalene to the Steroid Nucleus
squalene monooxygenase adds one O atom from O2 to the end of the squalene chain, forming squalene 2,3-epoxide
requires NADPH
a mixed-function oxidase: the other O atom is reduced by NADPH to H2O
the double bonds of squalene 2,3-epoxide are positioned in a way that can convert the linear squalene epoxide to a cyclic structure
this cyclization results in lanosterol, which contains the 4 ring steroid nucleus
lanosterol is converted to cholesterol in a series of 20 rxns
Stage 4: Conversion of Squalene to the Steroid Nucleus FIGURE
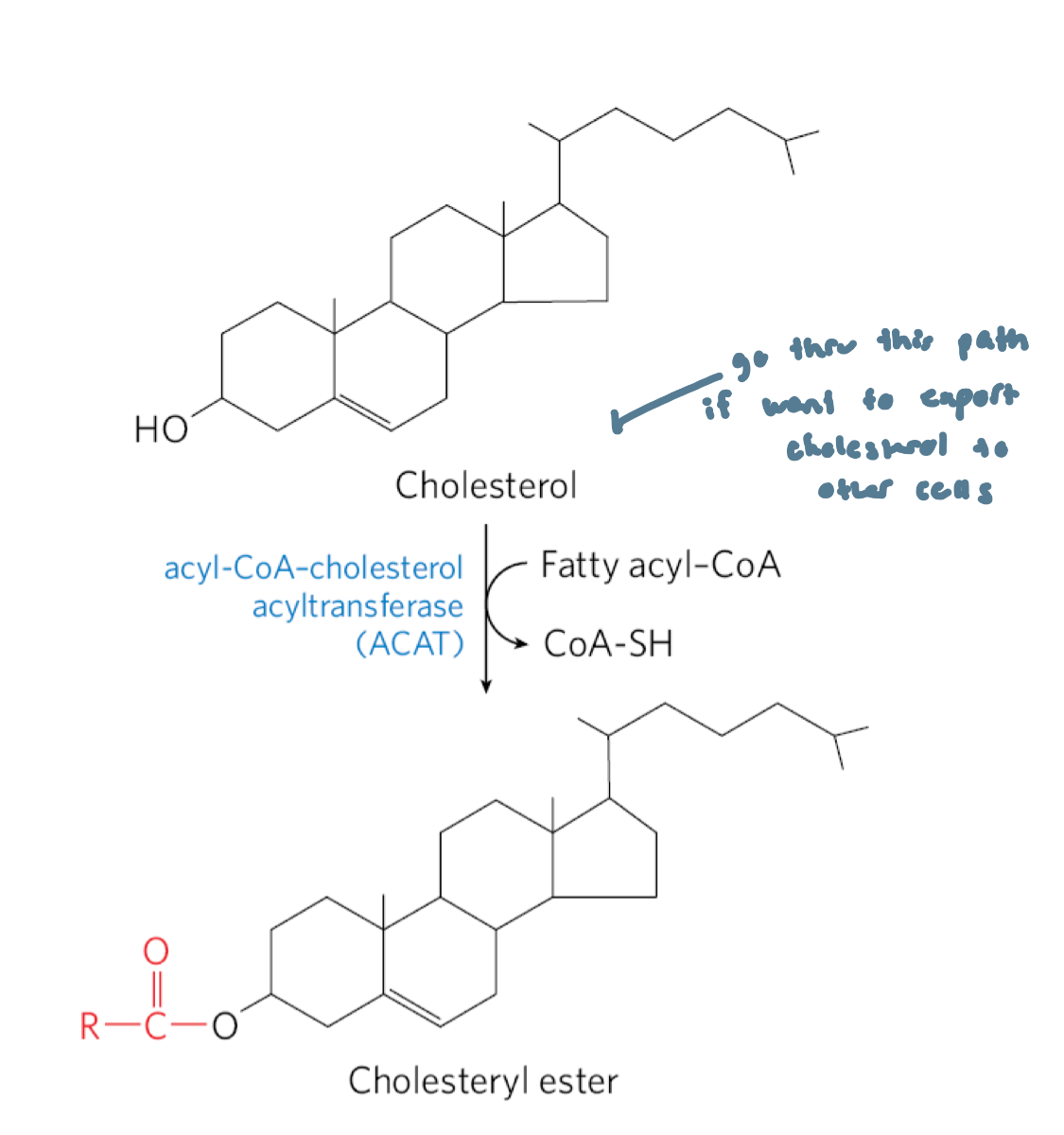
Several Fates of Cholesterol
cholesterol is primarily synthesized in the liver
a small fraction of cholesterol made there is incorporated into the membranes of hepatocytes
most of it is exported as: bile acids, biliary cholesterol or cholesteryl esters
cholesteryl esters are formed in the liver by acyl-CoA cholesterol acyltransferase (ACAT)
in other tissues, cholesterol is converted into steroid hormones (in adrenal cortex and gonads) or into vitamin D hormone (liver and kidney)
these hormones are biological signals acting thru nuclear receptor proteins
Bile Acids
fluid stored in the gallbladder and excreted into the small intestine to aid in the digestion of fatty meals
relatively hydrophobic cholesterol derivatives that serve as emulsifiers in the intestine
bile contains small amounts of cholesterol (biliary cholesterol)
helps remove excess cholesterol from the intestine and facilitates excretion
Dietary Fibers on Bile
can enhance the excess cholesterol effect by binding to bile and interfering with bile reabsorption in the intestines, leading to increased bile excretion in the feces
more cholesterol is then used to make bile
available in oats and barley
Acyl-CoA Cholesterol Acyltransferase (ACAT)
catalyzes the synthesis of cholesteryl esters in the liver
catalyzes the transfer of a fatty acid from coenzyme A to the hydroxyl group of cholesterol
this converts cholesterol into a more hydrophobic form that isn’t sufficiently amphipathic anymore to function appropriately in membranes
cholesteryl esters are transported in secreted lipoprotein particles to other tissues that use cholesterol, or are stored in the liver in lipid droplets
Reverse Cholesterol Pathway
HDL’s contain lecithin-cholesterol acyltransferase (LCAT) which catalyzes the formation of cholesteryl esters from lecithin (phosphatidylcholine) and cholesterol
HDL picks up cholesterol and cholesteryl-esters from cholesterol-rich extrahepatic cells and returns it to the liver for unloading
much of this cholesterol is converted to bile salts → stored in gallbladder
Why is Reverse Cholesterol Transport Useful?
HDL can get cholesterol from LDL and macrophages that”
don’t have access to liver/ aren’t brought back to liver
circulating LDL isn’t good, as it gets eaten up by macrophages, which break down cholesteryl-esters into cholesterol
cholesterol crystallizes and becomes plaque
when HDL’s are empty again, they can go thru the system again and get more cholesterol
Reverse Cholesterol Transport: Step 1
HDLs are synthesized, containing LCAT
LCAT on surfaces of nascent (newly forming) HDL particles converts the cholesterol and phosphatidylcholine of chylomicron and VLDL remnants to cholesteryl esters
this begins to form a core, transforming the disk-shaped nascent HDL to a mature, spherical HDL particle
Reverse Cholesterol Transport: Step 2
Nascent HDL can also pick up cholesterol from cholesterol-rich extrahepatic cells
including macrophages, and foam cells (formed from macrophages)
Reverse Cholesterol Transport: Step 3
Mature HDL then returns to the liver, where the cholesterol is unloaded via the scavenger receptor SR-BI
Reverse Cholesterol Transport: Step 4
Some of the cholesteryl esters in HDL can be transferred to LDL by the cholesteryl ester transfer protein
Receptor Mediated Endocytosis: Details
each LDL particle in the bloodstream contains apoB-100, which is recognized by LDL receptors present in the plasma membranes of cells that need to take up cholesterol
LDL receptors: receptors in the hepatocyte plasma membrane that take up LDL not taken up by peripheral tissues and cells
synthesized in the ER and modified by Golgi complex, then transported to the plasma membrane
ApoB-100 is also present in VLDL, but its receptor binding domain is not available for binding to the LDL receptor
conversion of VLDL to LDL exposes the receptor-binding domain of apoB-100
Receptor Mediated Endocytosis: Steps
LDL receptors are synthesized and transported to plasma membrane, where they are available to bind apoB-100
binding of LDL to an LDL receptor initiates endocytosis
this conveys LDL and its receptor into the cell within an endosome
the receptor containing portions of the endosome membrane bud off and are returned to the cell surface, to function again in LDL uptake
the endosome fuses with a lysosome which contains enzymes that hydrolyze the cholesteryl esters
this releases cholesterol and fatty acids into the cytosol
the apoB-100 is also degraded to amino acids that are released to the cytosol
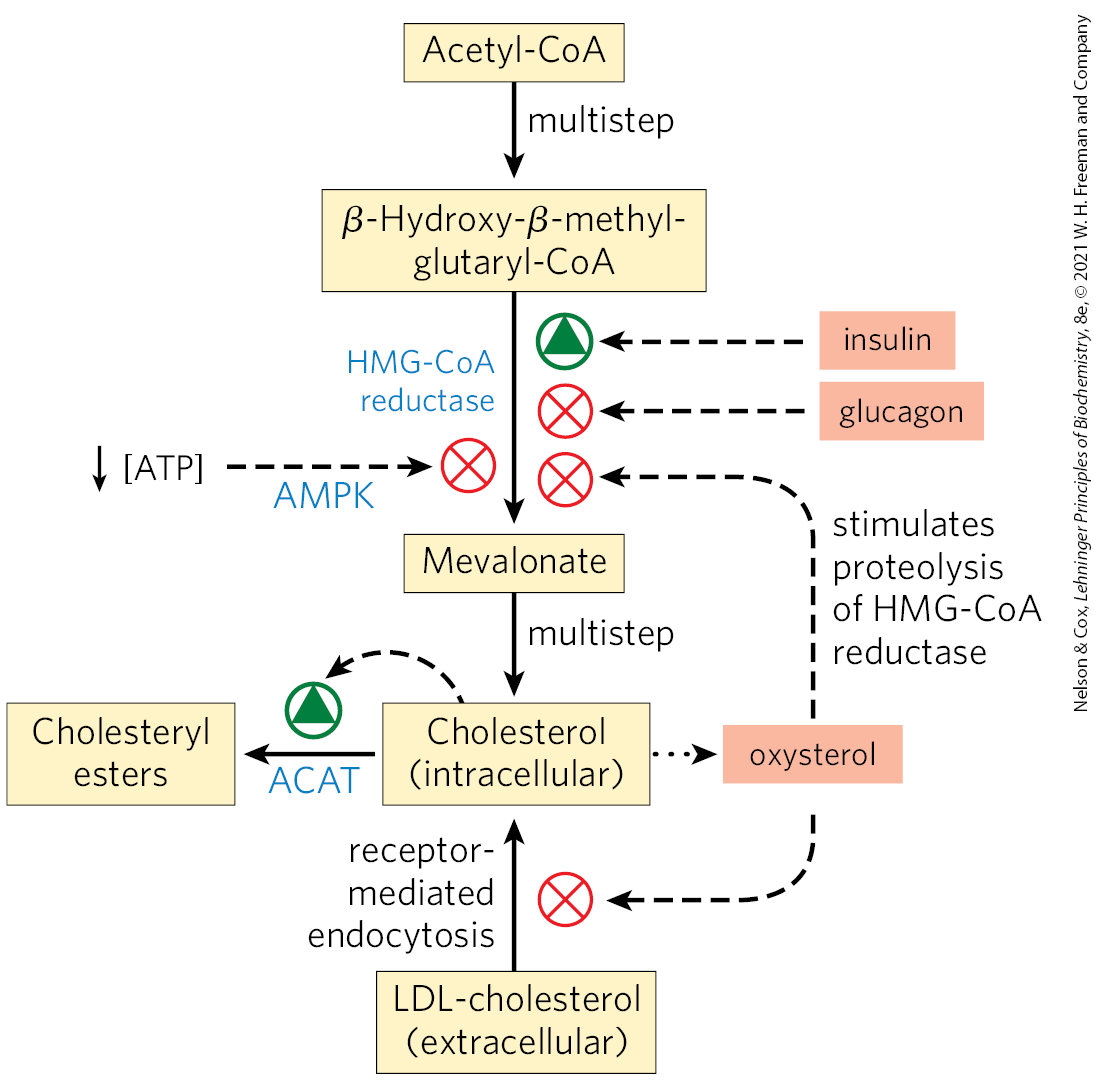
Cholesterol Synthesis/Transport Regulation
cholesterol synthesis is a complex and energy expensive process
excess cholesterol cannot be catabolized for use as fuel and must be excreted
thus advantageous to regulate the biosynthesis of cholesterol to complement dietary intake
in mammals, cholesterol production is regulated by intracellular cholesterol concentration, supply of ATP and by glucagon and insulin
the committed step in the pathway to cholesterol is HMG-CoA → mevalonate
Cholesterol Synthesis/Transport Regulation: Short-Term
Minute to minute
short term regulation of existing HMG-CoA reductase activity is accomplished by reversible covalent alteration
phosphorylation by the AMP-dependent protein kinase (AMPK), which senses high AMP conc. (low ATP conc.)
thus when ATP levels drop, the synthesis of cholesterol slows and catabolic pathways for the generation of ATP are stimulated
hormones also act on HMG-CoA: glucagon stimulates its phosphorylation (inactivation), insulin promotes dephosphorylation (activation, favoring cholesterol synthesis)
high intracellular concs. of cholesterol reduce transcription of the genes encoding HMG-CoA reductase and promote its proteolytic degradation.
Dysregulation of Cholesterol Metabolism
can lead to cardiovascular disease
excess cholesterol can be removed only by excretion or by conversion to bile salts
atherosclerosis: the obstruction of blood vessels from the pathological accumulation of cholesterol (plaques)
linked to high levels of cholesterol and LDL
macrophages can’t limit their uptake of sterols and with increasing accumulation of cholesteryl esters and free cholesterol, they become foam cells
overtime, the cells undergo apoptosis (cell death)
Plaque from Cholesterol
initiated when LDL containing partially oxidized fatty acyl groups adheres to and accumulates in the extracelluar matrix of epithelial cells lining arteries
over time, arteries become progressively occluded as plaques consisting of extracellular matrix material, scar tissue and foam cell remnants gradually grow larger
within the cholesterol-rich plaques, cholesterol can crystallize
occasionally, a plaque breaks loose from the site of its formation and is carried thru the blood to a narrowed region of an artery in the brain or heart, causing a stroke or heart attack
or cause vascular injury
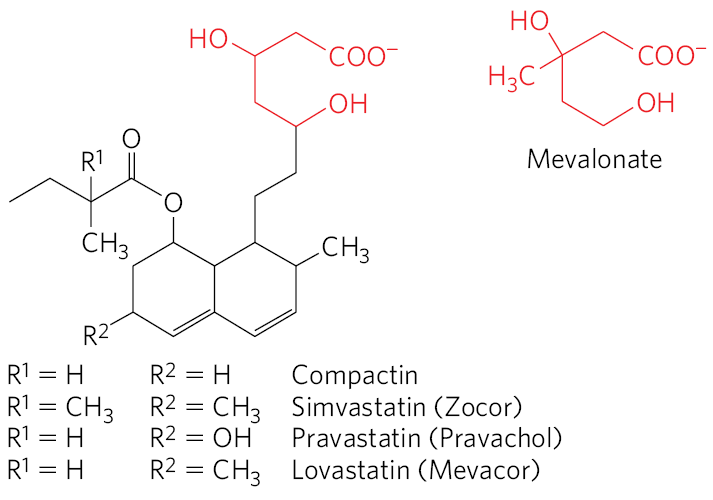
Statins
drug class used to treat patients with elevated serum cholesterol
resembled mevalonate
competitive inhibitors of HMG-CoA reductase
prevents things downstream of mevalonate
HMG-CoA Reductase step is a good step to target for restricting cholesterol synthesis because it’s the commitment step
Familial Hypercholesterolemia
characterized by extremely high blood levels of cholesterol due to a defective LDL receptor
this prevents the normal uptake of LDL by liver and peripheral tissues, resulting in high blood levels of LDL and of the cholesterol it carries
cholesterol accumulates in foam cells and contributes to the formation of atherosclerotic plaques
increased probability of developing atherosclerosis
Reverse Cholesterol Transport
process by which HDL removes cholesterol from peripheral tissues and carries it to the liver, protecting against atherosclerosis (reducing the potential damage from foam cell buildup)
cholesterol movement out of cells requires transporters.
ApoA-I interacts with ABCA1 transporter in a cholesterol rich cell
ABCA1 transports a load of cholesterol from inside the cell to the outer surface of the plasma membrane, where lipid-free/-poor apoA-I picks it up, then transporting it to the liver
another transporter, ABCG1 interacts with mature HDL, facilitating the movement of cholesterol out of the cell and into the HDL
As HDL matures, it gains more cholesterol (with ABCG1 also helping)
the resulting HDL particle then transports it to the liver
Reverse Cholesterol Transport FIGURE
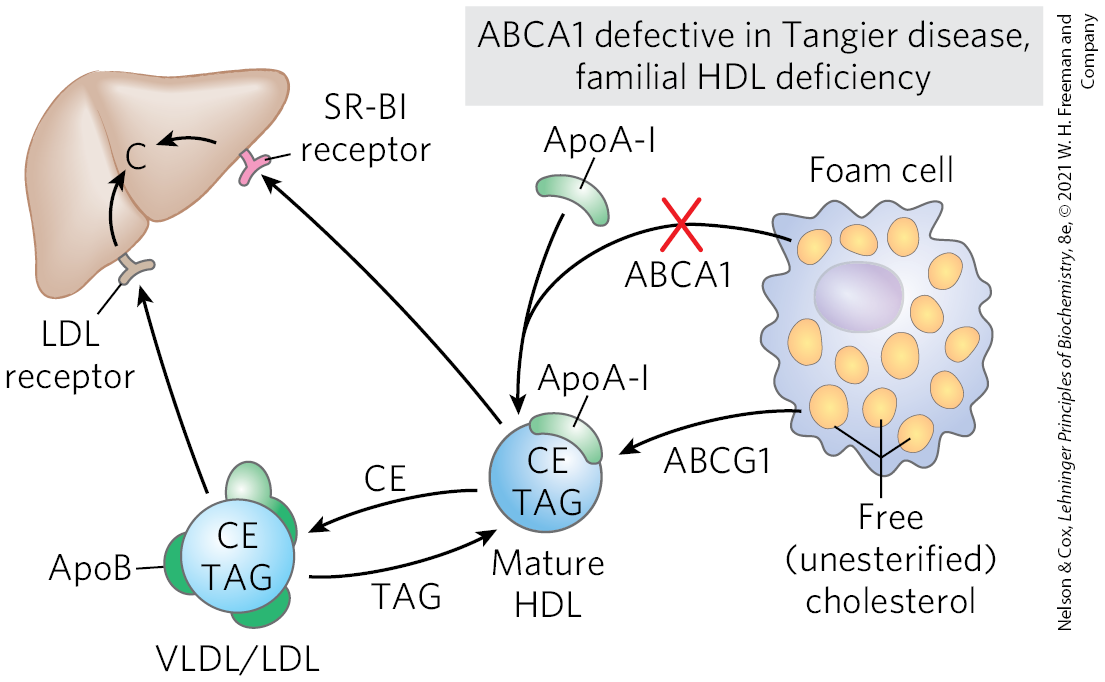
Familial HDL Deficiency and Tangier Disease
Familial HDL Deficiency: HDL levels are very low
Tangier Disease: HDL levels are almost undetectable
both genetic disorders are the result of mutations in the ABCA1 protein
ApoA-I in cholesterol-depleted HDL cannot take up cholesterol from cells that lack ABCA1 protein, and apoA-I and cholesterol-poor HDL are rapidly removed from the blood and destroyed
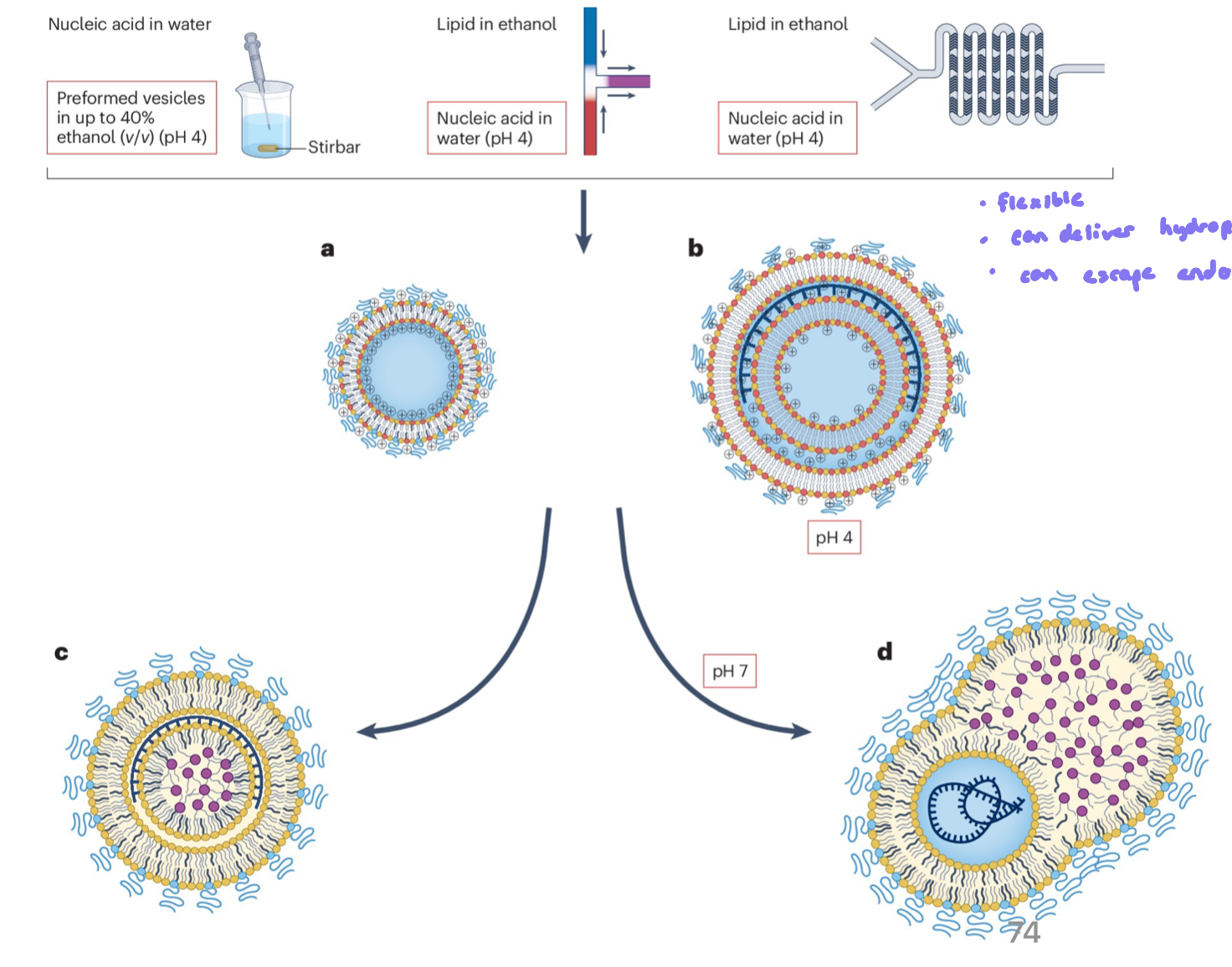
Lipid Nanoparticles (LNP)
allow for the transport of negatively charged nucleic acid polymers thru the body to the target tissue (easiest ones currently are blood cells and liver, but this is rapidly advancing)
different structural lipids have distinct properties and propensities for forming lipid structures, these can be leveraged to carry different types of cargo
pH dependent ionization of structural lipid head groups enables the uptake of the nucleic acid, as well as release during endocytosis
we are using these particles in lots of interesting ways to deliver drugs and therapeutics
flexible and can escape endosomes
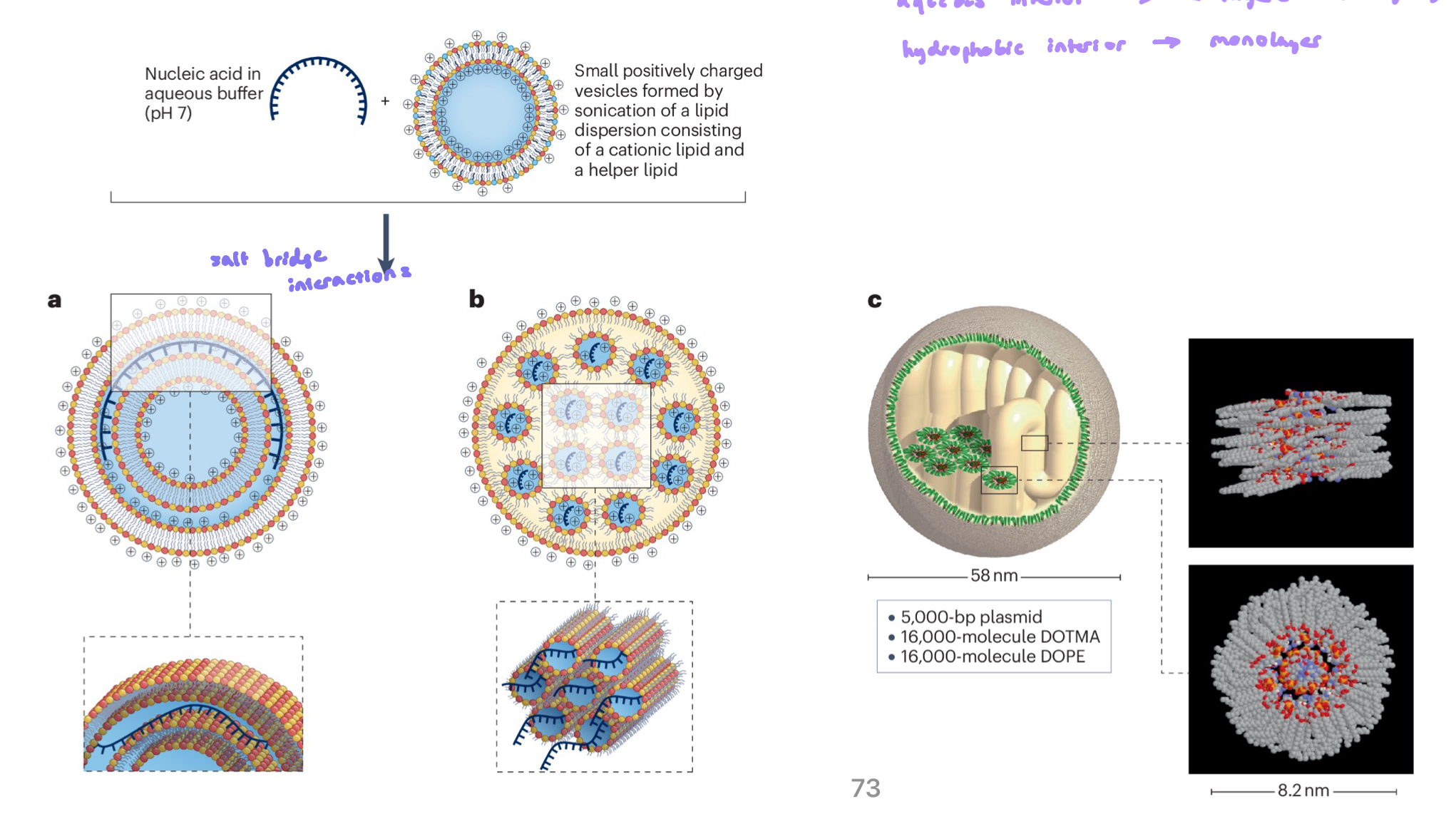
Formation and Structure of a Lipoplex
involves the electrostatic attraction and binding of anionic nucleic acids (like DNA) to cationic lipid vesicles
process driven by the + charge of the lipid headgroups and - charged DNA backbone
the strong attraction causes lipid bilayers to cluster together (aggregate) and sometimes disrupt (rupture) because the nucleic acid inserts b/w them
the result is a layered (multiamellar) structure, where sheets of lipid alternate with layers of condensed nucleic acid, like a sandwich
If you want:
aqueous interior → bilayer
hydrophobic interior → monolayer
Formation of Lipid Nano Particle
lipids are dissolved in an organic solvent (eg. ethanol)
mixed rapidly with an aqueous solution containing nucleic acids (eg. mRNA)
rapid mixing triggers self-assembly into LNPs
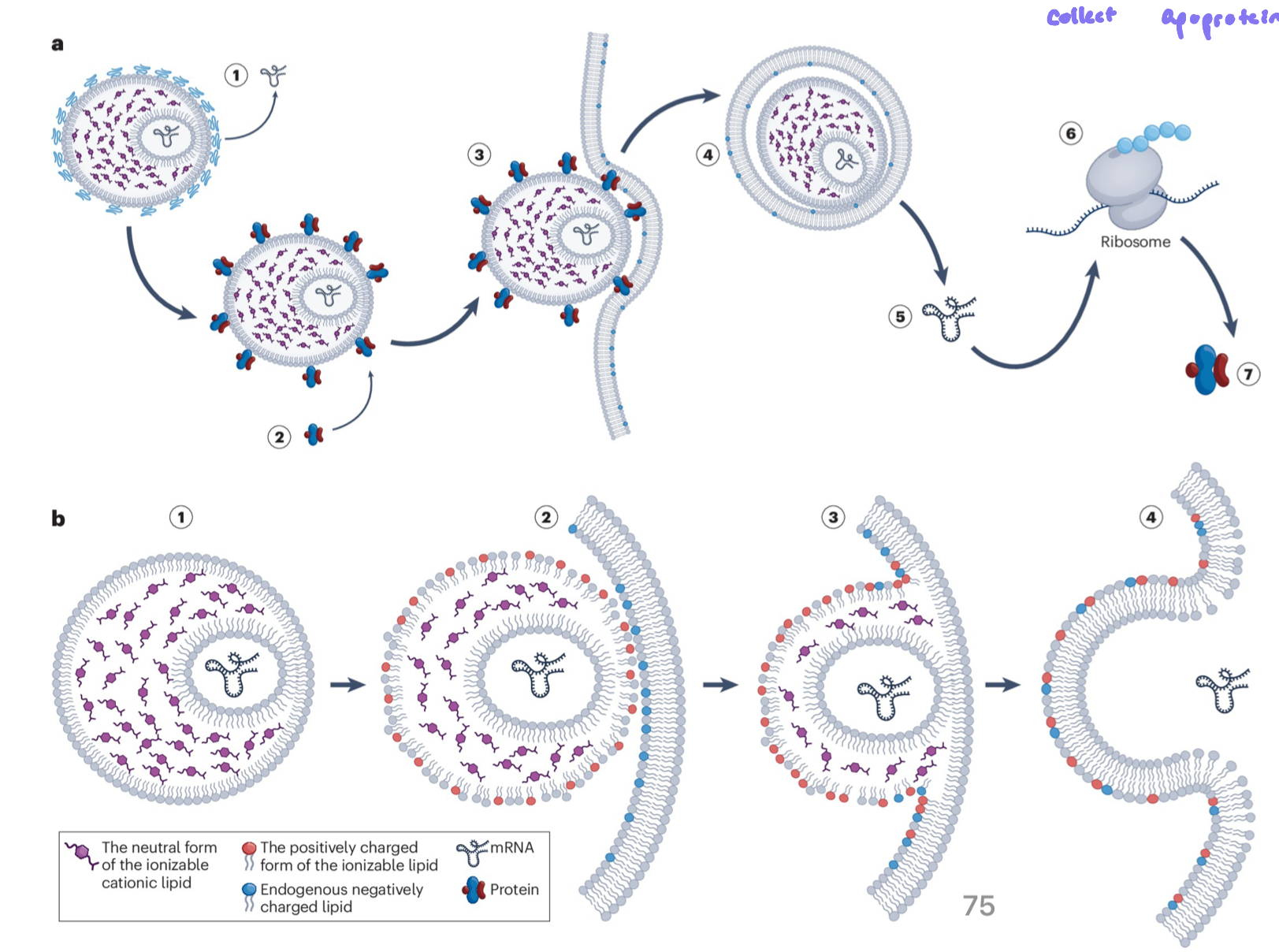
Mechanism of LNP mRNA Deliver
LNPs carrying mRNA are introduced (eg. by injection)
cells take up LNPs by endocytosis
LNPs disrupt the endosome membrane via pH ionization, releasing mRNA into the cytoplasm
released mRNA is translated into protein by ribosomes
siRNA to target PCSK9
siRNA is a drug therapy, treating high cholesterol
reduces LDL cholesterol by inhibiting PCSK9 protein production in the liver
siRNA enters liver cells often via LNPs
in the cytoplasm, siRNA incorporates into the RISC complex
RISC-siRNA complex binds PCSK9 mRNA, causing mRNA degradation
reduced PCSK9 protein increases LDL receptor availability on hepatocytes, resulting in more LDL cholesterol cleared from the blood
Normal PCSK9 Activity
PCSK9 directs LDL receptors to lysosomes, where they are degraded and can’t return to the cell surface
this leads to less cholesterol being cleared form the blood