Biochem HW 1 (Chapters 2-4)
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
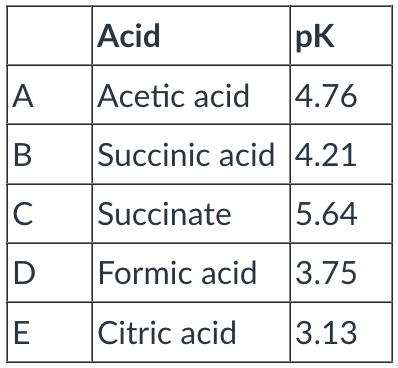
Rank the following according to their strength as acids--rank from strongest (1) to weakest (5).
A: 4
B: 3
C: 5
D: 2
E: 1
Which of the following would NOT form a suitable buffer?
Hydrochloric acid/chloride
Under what conditions would a carboxyl group (COOH, pK 3.5) be mostly protonated?
pH < pK
Under what conditions would a carboxyl group (COOH, pK 3.5) be mostly deprotonated?
pH > pK
In aqueous solution, globules of up to several thousand amphiphilic molecules arranged with the hydrophilic groups on the surface and the hydrophobic groups buried in the center are called _____.
Micelles
What term is used to describe the exclusion of nonpolar substances from an aqueous solution?
Hydrophobic effect
Which of the following is an example of the hydrophobic effect?
all are examples of the hydrophobic effect.
A molecule that has both a polar and nonpolar region is called _____________.
Amphiphilic
If the pK value for acetic acid (CH3COOH) is 4.76 at what pH would one observe equal amounts of CH3COO- and CH3COOH?
4.76
T/F: The LOWER the pK value of an acid the STRONGER the acid.
True
A buffer contains 0.050M of lactic acid (pK= 3.86) and 0.010M of lactate per liter. Use the Henderson-Hasselbalch equation to calculate the pH of the buffer. Enter your answer to two decimal points.
3.16